Long term natural history data in ambulant boys with Duchenne muscular dystrophy: 36-month changes
- PMID: 25271887
- PMCID: PMC4182715
- DOI: 10.1371/journal.pone.0108205
Long term natural history data in ambulant boys with Duchenne muscular dystrophy: 36-month changes
Erratum in
-
Correction: Long term natural history data in ambulant boys with Duchenne muscular dystrophy: 36-month changes.PLoS One. 2015 Mar 25;10(3):e0121882. doi: 10.1371/journal.pone.0121882. eCollection 2015. PLoS One. 2015. PMID: 25806823 Free PMC article. No abstract available.
-
Correction: Long Term Natural History Data in Ambulant Boys with Duchenne Muscular Dystrophy: 36-Month Changes.PLoS One. 2015 Dec 4;10(12):e0144079. doi: 10.1371/journal.pone.0144079. eCollection 2015. PLoS One. 2015. PMID: 26636671 Free PMC article. No abstract available.
Abstract
The 6 minute walk test has been recently chosen as the primary outcome measure in international multicenter clinical trials in Duchenne muscular dystrophy ambulant patients. The aim of the study was to assess the spectrum of changes at 3 years in the individual measures, their correlation with steroid treatment, age and 6 minute walk test values at baseline. Ninety-six patients from 11 centers were assessed at baseline and 12, 24 and 36 months after baseline using the 6 minute walk test and the North Star Ambulatory Assessment. Three boys (3%) lost the ability to perform the 6 minute walk test within 12 months, another 13 between 12 and 24 months (14%) and 11 between 24 and 36 months (12%). The 6 minute walk test showed an average overall decline of -15.8 (SD 77.3) m at 12 months, of -58.9 (SD 125.7) m at 24 months and -104.22 (SD 146.2) m at 36 months. The changes were significantly different in the two baseline age groups and according to the baseline 6 minute walk test values (below and above 350 m) (p<0.001). The changes were also significantly different according to steroid treatment (p = 0.01). Similar findings were found for the North Star Ambulatory Assessment. These are the first 36 month longitudinal data using the 6 minute walk test and North Star Ambulatory Assessment in Duchenne muscular dystrophy. Our findings will help not only to have a better idea of the progression of the disorder but also provide reference data that can be used to compare with the results of the long term extension studies that are becoming available.
Conflict of interest statement
Figures

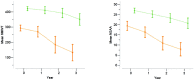
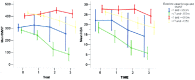
References
-
- McDonald CM, Henricson EK, Abresch RT, Han JJ, Escolar DM, et al. (2013) The cooperative international neuromuscular research group Duchenne natural history study–a longitudinal investigation in the era of glucocorticoid therapy: design of protocol and the methods used. Muscle Nerve 48: 32–54. - PMC - PubMed
-
- McDonald CM, Henricson EK, Abresch RT, Florence J, Eagle M, et al. (2013) The 6-minute walk test and other clinical endpoints in duchenne muscular dystrophy: Reliability, concurrent validity, and minimal clinically important differences from a multicenter study. Muscle Nerve 48: 343–356. - PMC - PubMed
-
- Henricson EK, Abresch RT, Cnaan A, Hu F, Duong T, et al. (2013) The cooperative international neuromuscular research group Duchenne natural history study: glucocorticoid treatment preserves clinically meaningful functional milestones and reduces rate of disease progression as measured by manual muscle testing and other commonly used clinical trial outcome measures. Muscle Nerve 48: 55–67. - PMC - PubMed
-
- Mayhew A, Mazzone ES, Eagle M, Duong T, Ash M, et al. (2013) Development of the Performance of the Upper Limb module for Duchenne muscular dystrophy. Dev Med Child Neurol 55: 1038–1045. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

