Rhinovirus-induced IL-25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation
- PMID: 25273095
- PMCID: PMC4246061
- DOI: 10.1126/scitranslmed.3009124
Rhinovirus-induced IL-25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation
Abstract
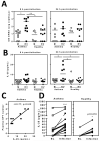
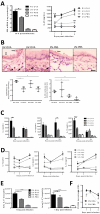
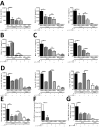
Rhinoviruses (RVs), which are the most common cause of virally induced asthma exacerbations, account for much of the burden of asthma in terms of morbidity, mortality, and associated cost. Interleukin-25 (IL-25) activates type 2-driven inflammation and is therefore potentially important in virally induced asthma exacerbations. To investigate this, we examined whether RV-induced IL-25 could contribute to asthma exacerbations. RV-infected cultured asthmatic bronchial epithelial cells exhibited a heightened intrinsic capacity for IL-25 expression, which correlated with donor atopic status. In vivo human IL-25 expression was greater in asthmatics at baseline and during experimental RV infection. In addition, in mice, RV infection induced IL-25 expression and augmented allergen-induced IL-25. Blockade of the IL-25 receptor reduced many RV-induced exacerbation-specific responses including type 2 cytokine expression, mucus production, and recruitment of eosinophils, neutrophils, basophils, and T and non-T type 2 cells. Therefore, asthmatic epithelial cells have an increased intrinsic capacity for expression of a pro-type 2 cytokine in response to a viral infection, and IL-25 is a key mediator of RV-induced exacerbations of pulmonary inflammation.
Copyright © 2014, American Association for the Advancement of Science.
Figures


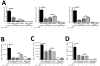
Comment in
-
IL-25: the missing link between allergy, viral infection, and asthma?Sci Transl Med. 2014 Oct 1;6(256):256fs38. doi: 10.1126/scitranslmed.3010273. Sci Transl Med. 2014. PMID: 25273094
-
Lung disease: IL-25 blockade could reduce virus-associated asthma attacks.Nat Rev Drug Discov. 2014 Nov;13(11):810-1. doi: 10.1038/nrd4473. Nat Rev Drug Discov. 2014. PMID: 25359375 No abstract available.
References
-
- Botturi K, Langelot M, Lair D, Pipet A, Pain M, Chesne J, Hassoun D, Lacoeuille Y, Cavailles A, Magnan A. Preventing asthma exacerbations: what are the targets? Pharmacol Ther. 2011;131:114–129. - PubMed
-
- Larche M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol. 2003;111:450–463. quiz 464. - PubMed
-
- Christodoulopoulos P, Cameron L, Nakamura Y, Lemiere C, Muro S, Dugas M, Boulet LP, Laviolette M, Olivenstein R, Hamid Q. TH2 cytokine-associated transcription factors in atopic and nonatopic asthma: evidence for differential signal transducer and activator of transcription 6 expression. J Allergy Clin Immunol. 2001;107:586–591. - PubMed
-
- Berry MA, Parker D, Neale N, Woodman L, Morgan A, Monk P, Bradding P, Wardlaw AJ, Pavord ID, Brightling CE. Sputum and bronchial submucosal IL-13 expression in asthma and eosinophilic bronchitis. J Allergy Clin Immunol. 2004;114:1106–1109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

