Cartilage repair using human embryonic stem cell-derived chondroprogenitors
- PMID: 25273540
- PMCID: PMC4214847
- DOI: 10.5966/sctm.2014-0101
Cartilage repair using human embryonic stem cell-derived chondroprogenitors
Abstract
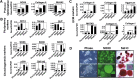
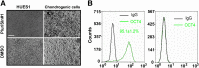
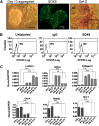
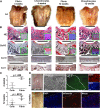
In initial work, we developed a 14-day culture protocol under potential GMP, chemically defined conditions to generate chondroprogenitors from human embryonic stem cells (hESCs). The present study was undertaken to investigate the cartilage repair capacity of these cells. The chondrogenic protocol was optimized and validated with gene expression profiling. The protocol was also applied successfully to two lines of induced pluripotent stem cells (iPSCs). Chondrogenic cells derived from hESCs were encapsulated in fibrin gel and implanted in osteochondral defects in the patella groove of nude rats, and cartilage repair was evaluated by histomorphology and immunocytochemistry. Genes associated with chondrogenesis were upregulated during the protocol, and pluripotency-related genes were downregulated. Aggregation of chondrogenic cells was accompanied by high expression of SOX9 and strong staining with Safranin O. Culture with PluriSln1 was lethal for hESCs but was tolerated by hESC chondrogenic cells, and no OCT4-positive cells were detected in hESC chondrogenic cells. iPSCs were also shown to generate chondroprogenitors in this protocol. Repaired tissue in the defect area implanted with hESC-derived chondrogenic cells was stained for collagen II with little collagen I, but negligible collagen II was observed in the fibrin-only controls. Viable human cells were detected in the repair tissue at 12 weeks. The results show that chondrogenic cells derived from hESCs, using a chemically defined culture system, when implanted in focal defects were able to promote cartilage repair. This is a first step in evaluating these cells for clinical application for the treatment of cartilage lesions.
Keywords: Arthritis; Cell transplantation; Embryonic stem cell; Tissue regeneration.
©AlphaMed Press.
Figures
References
-
- Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. - PubMed
-
- Filardo G, Kon E, Berruto M, et al. Arthroscopic second generation autologous chondrocytes implantation associated with bone grafting for the treatment of knee osteochondritis dissecans: Results at 6 years. Knee. 2012;19:658–663. - PubMed
-
- Kang SW, Yoo SP, Kim BS. Effect of chondrocyte passage number on histological aspects of tissue-engineered cartilage. Biomed Mater Eng. 2007;17:269–276. - PubMed
-
- Hettrich CM, Crawford D, Rodeo SA. Cartilage repair: Third-generation cell-based technologies: Basic science, surgical techniques, clinical outcomes. Sports Med Arthrosc. 2008;16:230–235. - PubMed
-
- Pelttari K, Winter A, Steck E, et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254–3266. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

