The carboxyl terminus of VEGF-A is a potential target for anti-angiogenic therapy
- PMID: 25274272
- PMCID: PMC4280485
- DOI: 10.1007/s10456-014-9444-3
The carboxyl terminus of VEGF-A is a potential target for anti-angiogenic therapy
Abstract
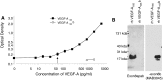
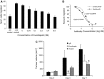
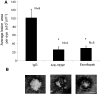
Anti-VEGF-A therapy has become a mainstay of treatment for ocular neovascularisation and in cancer; however, their effectiveness is not universal, in some cases only benefiting a minority of patients. Anti-VEGF-A therapies bind and block both pro-angiogenic VEGF-Axxx and the partial agonist VEGF-Axxxb isoforms, but their anti-angiogenic benefit only comes about from targeting the pro-angiogenic isoforms. Therefore, antibodies that exclusively target the pro-angiogenic isoforms may be more effective. To determine whether C-terminal-targeted antibodies could inhibit angiogenesis, we generated a polyclonal antibody to the last nine amino acids of VEGF-A165 and tested it in vitro and in vivo. The exon8a polyclonal antibody (Exon8apab) did not bind VEGF-A165b even at greater than 100-fold excess concentration, and dose dependently inhibited VEGF-A165 induced endothelial migration in vitro at concentrations similar to the VEGF-A antibody fragment ranibizumab. Exon8apab can inhibit tumour growth of LS174t cells implanted in vivo and blood vessel growth in the eye in models of age-related macular degeneration, with equal efficacy to non-selective anti-VEGF-A antibodies. It also showed that it was the VEGF-Axxx levels specifically that were upregulated in plasma from patients with proliferative diabetic retinopathy. These results suggest that VEGF-A165-specific antibodies can be therapeutically useful.
Figures

References
-
- Artac RA, McFee RM, Smith RAL, Baltes-Breitwisch MM, Clopton DT, Cupp AS. Neutralization of vascular endothelial growth factor antiangiogenic isoforms is more effective than treatment with proangiogenic isoforms in stimulating vascular development and follicle progression in the perinatal rat ovary. Biol Reprod. 2009;81(5):978–988. doi: 10.1095/biolreprod.109.078097. - DOI - PMC - PubMed
-
- Bates DO, Catalano PJ, Symonds KE, Varey AHR, Ramani P, O’Dwyer PJ, Giantonio BJ, Meropol NJ, Benson AB, Harper SJ. Association between VEGF splice isoforms and progression-free survival in metastatic colorectal cancer patients treated with bevacizumab. Clin Cancer Res. 2012;18(22):6384–6391. doi: 10.1158/1078-0432.CCR-12-2223. - DOI - PMC - PubMed
-
- Bates DO, Cui TG, Doughty JM, Winkler M, Sugiono M, Shields JD, Peat D, Gillatt D, Harper SJ. VEGF(165)b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 2002;62(14):4123–4131. - PubMed
-
- Bates DO, MacMillan PP, Manjaly JG, Qiu Y, Hudson SJ, Bevan HS, Hunter AJ, Soothill PW, Read M, Donaldson LF, Harper SJ. The endogenous anti-angiogenic family of splice variants of VEGF, VEGF(xxx)b, are down-regulated in pre-eclamptic placentae at term. Clin Sci. 2006;110(5):575–585. doi: 10.1042/CS20050292. - DOI - PubMed
-
- Bhattacharjee G, Asplin IR, Wu SM, Gawdi G, Pizzo SV. The conformation-dependent interaction of alpha(2)-macroglobulin with vascular endothelial growth factor—a novel mechanism of alpha(2)-macroglobulin/growth factor binding. J Biol Chem. 2000;275(35):26806–26811. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

