Reliability and extension of quantitative prediction of CYP3A4-mediated drug interactions based on clinical data
- PMID: 25274605
- PMCID: PMC4389753
- DOI: 10.1208/s12248-014-9663-y
Reliability and extension of quantitative prediction of CYP3A4-mediated drug interactions based on clinical data
Abstract
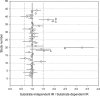
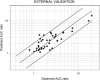
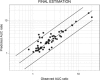
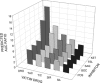
An approach was proposed in 2007 for quantitative predictions of cytochrome P450 (CYP)3A4-mediated drug-drug interactions. It is based on two characteristic parameters: the contribution ratio (CR; i.e., the fraction of victim drug clearance by CYP) and the inhibition ratio (IR) of the inhibitor. Knowledge of these parameters allows forecasting of the ratio between the area under the plasma concentration-time curve (AUC) of the victim drug when given with the inhibitor and the AUC of the victim drug when it is given alone. So far, these parameters were established for 21 substrates and 17 inhibitors. The goals of our study were to test the assumption of substrate independence of the potency of inhibitors in vivo and to estimate the CR and IR for an extended list of substrates and inhibitors of CYP3A4. The assumption of independence of IRs from the substrate was evaluated on a set of eight victim drugs and eight inhibitors. Forty-four AUC ratios were available. This assumption was rejected in four cases, but it did not result in more than a twofold error in AUC ratio predictions. The extended list of substrates and inhibitors was defined by a thorough literature search. Fifty-nine AUC ratios were available for the global analysis. Final estimates of CRs and IRs were obtained for 37 substrates and 25 inhibitors, respectively. The mean prediction error of the ratios was 0.02, while the mean absolute prediction error was 0.58. Predictive distributions for 917 possible interactions were obtained, giving detailed information on some drugs or inhibitors that have been poorly studied so far.
Figures
References
-
- Examples of sensitive in vivo CYP substrates and CYP substrates with narrow therapeutic range (7/28/2011) [Internet]. Available from http://www.fda.gov/drugs/developmentapprovalprocess/developmentresources.... Accessed 29 Jul 2014
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

