Therapeutic strategies to inhibit MYC
- PMID: 25274755
- PMCID: PMC4200208
- DOI: 10.1101/cshperspect.a014266
Therapeutic strategies to inhibit MYC
Abstract
MYC is a master regulator of stem cell state, embryogenesis, tissue homeostasis, and aging. As in health, in disease MYC figures prominently. Decades of biological research have identified a central role for MYC in the pathophysiology of cancer, inflammation, and heart disease. The centrality of MYC to such a vast breadth of disease biology has attracted significant attention to the historic challenge of developing inhibitors of MYC. This review will discuss therapeutic strategies toward the development of inhibitors of MYC-dependent transcriptional signaling, efforts to modulate MYC stability, and the elusive goal of developing potent, direct-acting inhibitors of MYC.
Copyright © 2014 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
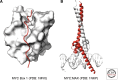

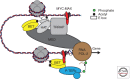
References
-
- Baell JB, Holloway GA. 2010. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J Med Chem 53: 2719–2740 - PubMed
-
- Berg T. 2008. Inhibition of transcription factors with small organic molecules. Curr Opin Chem Biol 12: 464–471 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
