Plasticity in developing brain: active auditory exposure impacts prelinguistic acoustic mapping
- PMID: 25274814
- PMCID: PMC6608311
- DOI: 10.1523/JNEUROSCI.0972-14.2014
Plasticity in developing brain: active auditory exposure impacts prelinguistic acoustic mapping
Abstract
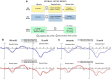
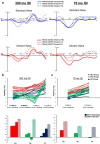
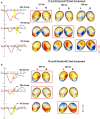
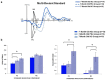
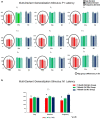
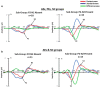
A major task across infancy is the creation and tuning of the acoustic maps that allow efficient native language processing. This process crucially depends on ongoing neural plasticity and keen sensitivity to environmental cues. Development of sensory mapping has been widely studied in animal models, demonstrating that cortical representations of the sensory environment are continuously modified by experience. One critical period for optimizing human language mapping is early in the first year; however, the neural processes involved and the influence of passive compared with active experience are as yet incompletely understood. Here we demonstrate that, while both active and passive acoustic experience from 4 to 7 months of age, using temporally modulated nonspeech stimuli, impacts acoustic mapping, active experience confers a significant advantage. Using event-related potentials (ERPs), we show that active experience increases perceptual vigilance/attention to environmental acoustic stimuli (e.g., larger and faster P2 peaks) when compared with passive experience or maturation alone. Faster latencies are also seen for the change discrimination peak (N2*) that has been shown to be a robust infant predictor of later language through age 4 years. Sharpening is evident for both trained and untrained stimuli over and above that seen for maturation alone. Effects were also seen on ERP morphology for the active experience group with development of more complex waveforms more often seen in typically developing 12- to 24-month-old children. The promise of selectively "fine-tuning" acoustic mapping as it emerges has far-reaching implications for the amelioration and/or prevention of developmental language disorders.
Keywords: EEG/ERP; acoustic mapping; developmental plasticity; human infant; prelinguistic; training.
Copyright © 2014 the authors 0270-6474/14/3413349-15$15.00/0.
Figures

References
-
- Benasich AA, Choudhury N, Friedman JT, Realpe-Bonilla T, Chojnowska C, Gou Z. The infant as a prelinguistic model for language learning impairments: predicting from event-related potentials to behavior. Neuropsychology. 2006;44:396–411. doi: 10.1016/j.neuropsychologia.2005.06.004. - DOI - PMC - PubMed
-
- Centanni TM, Booker AB, Sloan AM, Chen F, Maher BJ, Carraway RS, Khodaparast N, Rennaker R, LoTurco JJ, Kilgard MP. Knockdown of the dyslexia-associated gene kiaa0319 impairs temporal responses to speech stimuli in rat primary auditory cortex. Cereb Cortex. 2014;24:1753–1766. doi: 10.1093/cercor/bht028. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
