Loss of Smarc proteins impairs cerebellar development
- PMID: 25274825
- PMCID: PMC6608313
- DOI: 10.1523/JNEUROSCI.2560-14.2014
Loss of Smarc proteins impairs cerebellar development
Abstract
SMARCA4 (BRG1) and SMARCB1 (INI1) are tumor suppressor genes that are crucially involved in the formation of malignant rhabdoid tumors, such as atypical teratoid/rhabdoid tumor (AT/RT). AT/RTs typically affect infants and occur at various sites of the CNS with a particular frequency in the cerebellum. Here, granule neurons and their progenitors represent the most abundant cell type and are known to give rise to a subset of medulloblastoma, a histologically similar embryonal brain tumor. To test how Smarc proteins influence the development of granule neurons and whether this population may serve as cellular origin for AT/RTs, we specifically deleted Smarca4 and Smarcb1 in cerebellar granule cell precursors. Respective mutant mice displayed severe ataxia and motor coordination deficits, but did not develop any tumors. In fact, they suffered from a severely hypoplastic cerebellum due to a significant inhibition of granule neuron precursor proliferation. Molecularly, this was accompanied by an enhanced activity of Wnt/β-catenin signaling that, by itself, is known to cause a nearly identical phenotype. We further used an hGFAP-cre allele, which deleted Smarcb1 much earlier and in a wider neural precursor population, but we still did not detect any tumor formation in the CNS. In summary, our results emphasize cell-type-dependent roles of Smarc proteins and argue against cerebellar granule cells and other progeny of hGFAP-positive neural precursors as the cellular origin for AT/RTs.
Keywords: AT/RT; Smarc; brain; cerebellum; development; tumor.
Copyright © 2014 the authors 0270-6474/14/3413486-06$15.00/0.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
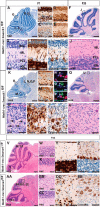

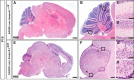
References
-
- Hasselblatt M, Gesk S, Oyen F, Rossi S, Viscardi E, Giangaspero F, Giannini C, Judkins AR, Frühwald MC, Obser T, Schneppenheim R, Siebert R, Paulus W. Nonsense mutation and inactivation of SMARCA4 (BRG1) in an atypical teratoid/rhabdoid tumor showing retained SMARCB1 (INI1) expression. Am J Surg Pathol. 2011;35:933–935. doi: 10.1097/PAS.0b013e3182196a39. - DOI - PubMed
-
- Hasselblatt M, Isken S, Linge A, Eikmeier K, Jeibmann A, Oyen F, Nagel I, Richter J, Bartelheim K, Kordes U, Schneppenheim R, Frühwald M, Siebert R, Paulus W. High-resolution genomic analysis suggests the absence of recurrent genomic alterations other than SMARCB1 aberrations in atypical teratoid/rhabdoid tumors. Genes Chromosomes Cancer. 2013;52:185–190. doi: 10.1002/gcc.22018. - DOI - PubMed
-
- Indra AK, Dupé V, Bornert JM, Messaddeq N, Yaniv M, Mark M, Chambon P, Metzger D. Temporally controlled targeted somatic mutagenesis in embryonic surface ectoderm and fetal epidermal keratinocytes unveils two distinct developmental functions of BRG1 in limb morphogenesis and skin barrier formation. Development. 2005;132:4533–4544. doi: 10.1242/dev.02019. - DOI - PubMed
-
- Jagani Z, Mora-Blanco EL, Sansam CG, McKenna ES, Wilson B, Chen D, Klekota J, Tamayo P, Nguyen PT, Tolstorukov M, Park PJ, Cho YJ, Hsiao K, Buonamici S, Pomeroy SL, Mesirov JP, Ruffner H, Bouwmeester T, Luchansky SJ, Murtie J, et al. Loss of the tumor suppressor Snf5 leads to aberrant activation of the Hedgehog-Gli pathway. Nat Med. 2010;16:1429–1433. doi: 10.1038/nm.2251. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
