Anterograde glycoprotein-dependent transport of newly generated rabies virus in dorsal root ganglion neurons
- PMID: 25275124
- PMCID: PMC4249153
- DOI: 10.1128/JVI.02254-14
Anterograde glycoprotein-dependent transport of newly generated rabies virus in dorsal root ganglion neurons
Abstract
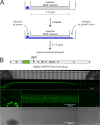
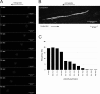
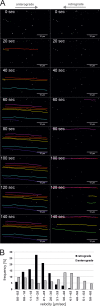
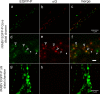
Rabies virus (RABV) spread is widely accepted to occur only by retrograde axonal transport. However, examples of anterograde RABV spread in peripheral neurons such as dorsal root ganglion (DRG) neurons indicated a possible bidirectional transport by an uncharacterized mechanism. Here, we analyzed the axonal transport of fluorescence-labeled RABV in DRG neurons by live-cell microscopy. Both entry-related retrograde transport of RABV after infection at axon endings and postreplicative transport of newly formed virus were visualized in compartmentalized DRG neuron cultures. Whereas entry-related transport at 1.5 μm/s occurred only retrogradely, after 2 days of infection, multiple particles were observed in axons moving in both the anterograde and retrograde directions. The dynamics of postreplicative retrograde transport (1.6 μm/s) were similar to those of entry-related retrograde transport. In contrast, anterograde particle transport at 3.4 μm/s was faster, indicating active particle transport. Interestingly, RABV missing the glycoproteins did not move anterogradely within the axon. Thus, anterograde RABV particle transport depended on the RABV glycoprotein. Moreover, colocalization of green fluorescent protein (GFP)-labeled ribonucleoproteins (RNPs) and glycoprotein in distal axonal regions as well as cotransport of labeled RNPs with membrane-anchored mCherry reporter confirmed that either complete enveloped virus particles or vesicle associated RNPs were transported. Our data show that anterograde RABV movement in peripheral DRG neurons occurs by active motor protein-dependent transport. We propose two models for postreplicative long-distance transport in peripheral neurons: either transport of complete virus particles or cotransport of RNPs and G-containing vesicles through axons to release virus at distal sites of infected DRG neurons.
Importance: Rabies virus retrograde axonal transport by dynein motors supports virus spread over long distances and lethal infection of the central nervous system. Though active rabies virus transport has been widely accepted to be unidirectional, evidence for anterograde spread in peripheral neurons supports the hypothesis that in some neurons RABV also enters the anterograde pathway by so-far unknown mechanisms. By live microscopy we visualized fast anterograde axonal transport of rabies virus. The velocities exceeded those of retrograde movements, suggesting that active, most likely kinesin-dependent transport machineries are involved. Dependency of anterograde transport on the expression of virus glycoprotein G and cotransport with vesicles further suggest that complete enveloped virus particles or cotransport of virus ribonucleoprotein and G-containing vesicles occurred. These data provide the first insight in the mechanism of anterograde rabies virus transport and substantially contribute to the understanding of RABV replication and spread of newly formed virus in peripheral neurons.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

