Natural isotopic signatures of variations in body nitrogen fluxes: a compartmental model analysis
- PMID: 25275306
- PMCID: PMC4183419
- DOI: 10.1371/journal.pcbi.1003865
Natural isotopic signatures of variations in body nitrogen fluxes: a compartmental model analysis
Abstract
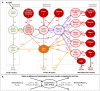
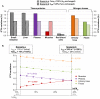
Body tissues are generally 15N-enriched over the diet, with a discrimination factor (Δ15N) that varies among tissues and individuals as a function of their nutritional and physiopathological condition. However, both 15N bioaccumulation and intra- and inter-individual Δ15N variations are still poorly understood, so that theoretical models are required to understand their underlying mechanisms. Using experimental Δ15N measurements in rats, we developed a multi-compartmental model that provides the first detailed representation of the complex functioning of the body's Δ15N system, by explicitly linking the sizes and Δ15N values of 21 nitrogen pools to the rates and isotope effects of 49 nitrogen metabolic fluxes. We have shown that (i) besides urea production, several metabolic pathways (e.g., protein synthesis, amino acid intracellular metabolism, urea recycling and intestinal absorption or secretion) are most probably associated with isotope fractionation and together contribute to 15N accumulation in tissues, (ii) the Δ15N of a tissue at steady-state is not affected by variations of its P turnover rate, but can vary according to the relative orientation of tissue free amino acids towards oxidation vs. protein synthesis, (iii) at the whole-body level, Δ15N variations result from variations in the body partitioning of nitrogen fluxes (e.g., urea production, urea recycling and amino acid exchanges), with or without changes in nitrogen balance, (iv) any deviation from the optimal amino acid intake, in terms of both quality and quantity, causes a global rise in tissue Δ15N, and (v) Δ15N variations differ between tissues depending on the metabolic changes involved, which can therefore be identified using simultaneous multi-tissue Δ15N measurements. This work provides proof of concept that Δ15N measurements constitute a new promising tool to investigate how metabolic fluxes are nutritionally or physiopathologically reorganized or altered. The existence of such natural and interpretable isotopic biomarkers promises interesting applications in nutrition and health.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Box-modeling of 15N/14N in mammals.Oecologia. 2006 Mar;147(2):212-22. doi: 10.1007/s00442-005-0263-5. Epub 2005 Dec 3. Oecologia. 2006. PMID: 16328553
-
Nitrogen isotopic composition in hair protein is different in liver cirrhotic patients.Rapid Commun Mass Spectrom. 2006;20(19):2973-8. doi: 10.1002/rcm.2695. Rapid Commun Mass Spectrom. 2006. PMID: 16955536
-
Fractionation and metabolic turnover of carbon and nitrogen stable isotopes in black fly larvae.Rapid Commun Mass Spectrom. 2008;22(5):694-700. doi: 10.1002/rcm.3413. Rapid Commun Mass Spectrom. 2008. PMID: 18257111
-
Nitrogen isotopes link mycorrhizal fungi and plants to nitrogen dynamics.New Phytol. 2012 Oct;196(2):367-382. doi: 10.1111/j.1469-8137.2012.04300.x. Epub 2012 Sep 11. New Phytol. 2012. PMID: 22963677 Review.
-
The in vivo nitrogen isotope discrimination among organic plant compounds.Phytochemistry. 2002 Nov;61(5):465-84. doi: 10.1016/s0031-9422(02)00204-2. Phytochemistry. 2002. PMID: 12409013 Review.
Cited by
-
Multi-species entanglements and stable isotope signals (δ13C and δ15N) in modern reindeer herding communities of boreal northeast Asia.Philos Trans R Soc Lond B Biol Sci. 2025 May;380(1926):20240203. doi: 10.1098/rstb.2024.0203. Epub 2025 May 15. Philos Trans R Soc Lond B Biol Sci. 2025. PMID: 40370022 Free PMC article.
-
Effect of Organic Food Intake on Nitrogen Stable Isotopes.Nutrients. 2020 Sep 28;12(10):2965. doi: 10.3390/nu12102965. Nutrients. 2020. PMID: 32998302 Free PMC article.
-
Changes in urinary stable nitrogen isotope ratios during controlled short-term energy deficit: a proof-of-principle analysis.Eur J Nutr. 2024 Apr;63(3):919-926. doi: 10.1007/s00394-023-03320-8. Epub 2024 Jan 19. Eur J Nutr. 2024. PMID: 38243136 Free PMC article.
-
Increase in stable isotope ratios driven by metabolic alterations in amphipods exposed to the beta-blocker propranolol.PLoS One. 2019 May 16;14(5):e0211304. doi: 10.1371/journal.pone.0211304. eCollection 2019. PLoS One. 2019. PMID: 31095563 Free PMC article.
-
'Trophic' and 'source' amino acids in trophic estimation: a likely metabolic explanation.Oecologia. 2017 Jun;184(2):317-326. doi: 10.1007/s00442-017-3881-9. Epub 2017 Jun 6. Oecologia. 2017. PMID: 28584941 Free PMC article. Review.
References
-
- Davis TA, Reeds PJ (2001) Of flux and flooding: the advantages and problems of different isotopic methods for quantifying protein turnover in vivo: II. Methods based on the incorporation of a tracer. Curr Opin Clin Nutr Metab Care 4: 51–56. - PubMed
-
- Fouillet H, Bos C, Gaudichon C, Tome D (2002) Approaches to quantifying protein metabolism in response to nutrient ingestion. J Nutr 132: 3208S–3218S. - PubMed
-
- Tessari P, Garibotto G (2000) Interorgan amino acid exchange. Curr Opin Clin Nutr Metab Care 3: 51–57. - PubMed
-
- Wagenmakers AJ (1999) Tracers to investigate protein and amino acid metabolism in human subjects. Proc Nutr Soc 58: 987–1000. - PubMed
-
- Reitsema LJ (2013) Beyond diet reconstruction: stable isotope applications to human physiology, health, and nutrition. Am J Hum Biol 25: 445–456. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

