Delivery of circulating lipoproteins to specific neurons in the Drosophila brain regulates systemic insulin signaling
- PMID: 25275323
- PMCID: PMC4210815
- DOI: 10.7554/eLife.02862
Delivery of circulating lipoproteins to specific neurons in the Drosophila brain regulates systemic insulin signaling
Abstract
The Insulin signaling pathway couples growth, development and lifespan to nutritional conditions. Here, we demonstrate a function for the Drosophila lipoprotein LTP in conveying information about dietary lipid composition to the brain to regulate Insulin signaling. When yeast lipids are present in the diet, free calcium levels rise in Blood Brain Barrier glial cells. This induces transport of LTP across the Blood Brain Barrier by two LDL receptor-related proteins: LRP1 and Megalin. LTP accumulates on specific neurons that connect to cells that produce Insulin-like peptides, and induces their release into the circulation. This increases systemic Insulin signaling and the rate of larval development on yeast-containing food compared with a plant-based food of similar nutritional content.
Keywords: D. melanogaster; blood brain barrier; developmental biology; insulin; lipoprotein; neuroscience; stem cells.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures
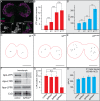
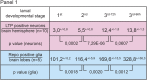



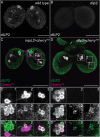

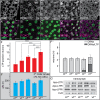






References
-
- Broughton SJ, Piper MD, Ikeya T, Bass TM, Jacobson J, Driege Y, Martinez P, Hafen E, Withers DJ, Leevers SJ, Partridge L. 2005. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proceedings of the National Academy of Sciences of USA 102:3105–3110. doi: 10.1073/pnas.0405775102 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

