Severe influenza A(H1N1)pdm09 infection induces thymic atrophy through activating innate CD8(+)CD44(hi) T cells by upregulating IFN-γ
- PMID: 25275588
- PMCID: PMC4649502
- DOI: 10.1038/cddis.2014.323
Severe influenza A(H1N1)pdm09 infection induces thymic atrophy through activating innate CD8(+)CD44(hi) T cells by upregulating IFN-γ
Abstract
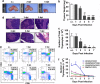
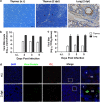
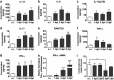
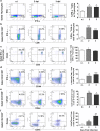
Thymic atrophy has been described as a consequence of infection by several pathogens including highly pathogenic avian influenza virus and is induced through diverse mechanisms. However, whether influenza A(H1N1)pdm09 infection induces thymic atrophy and the mechanisms underlying this process have not been completely elucidated. Our results show that severe infection of influenza A(H1N1)pdm09 led to progressive thymic atrophy and CD4+ CD8+ double-positive (DP) T-cells depletion due to apoptosis. The viruses were present in thymus, where they activated thymic innate CD8(+)CD44(hi) single-positive (SP) thymocytes to secrete a large amount of IFN-γ. Milder thymic atrophy was observed in innate CD8+ T-cell-deficient mice (C57BL/6J). Neutralization of IFN-γ could significantly rescue the atrophy, but peramivir treatment did not significantly alleviate thymic atrophy. In this study, we demonstrated that thymic innate CD8(+)CD44(hi) SP T-cells have critical roles in influenza A(H1N1)pdm09 infection-induced thymic atrophy through secreting IFN-γ. This exceptional mechanism might serve as a target for the prevention and treatment of thymic atrophy induced by influenza A(H1N1)pdm09.
Figures



Similar articles
-
Anti-IFN-γ therapy alleviates acute lung injury induced by severe influenza A (H1N1) pdm09 infection in mice.J Microbiol Immunol Infect. 2021 Jun;54(3):396-403. doi: 10.1016/j.jmii.2019.07.009. Epub 2019 Nov 12. J Microbiol Immunol Infect. 2021. PMID: 31780358
-
Induction of thymic atrophy and loss of thymic output by type-I interferons during chronic viral infection.Virology. 2022 Feb;567:77-86. doi: 10.1016/j.virol.2021.12.007. Epub 2021 Dec 24. Virology. 2022. PMID: 35032866
-
SARS-CoV-2 infection induces thymic atrophy mediated by IFN-γ in hACE2 transgenic mice.Eur J Immunol. 2024 Jul;54(7):e2350624. doi: 10.1002/eji.202350624. Epub 2024 Apr 24. Eur J Immunol. 2024. PMID: 38655818
-
When the Damage Is Done: Injury and Repair in Thymus Function.Front Immunol. 2020 Aug 12;11:1745. doi: 10.3389/fimmu.2020.01745. eCollection 2020. Front Immunol. 2020. PMID: 32903477 Free PMC article. Review.
-
Thymus Gland: A Double Edge Sword for Coronaviruses.Vaccines (Basel). 2021 Oct 2;9(10):1119. doi: 10.3390/vaccines9101119. Vaccines (Basel). 2021. PMID: 34696231 Free PMC article. Review.
Cited by
-
Recirculating IL-1R2+ Tregs fine-tune intrathymic Treg development under inflammatory conditions.Cell Mol Immunol. 2021 Jan;18(1):182-193. doi: 10.1038/s41423-019-0352-8. Epub 2020 Jan 27. Cell Mol Immunol. 2021. PMID: 31988493 Free PMC article.
-
Infection-Associated Thymic Atrophy.Front Immunol. 2021 May 25;12:652538. doi: 10.3389/fimmu.2021.652538. eCollection 2021. Front Immunol. 2021. PMID: 34113341 Free PMC article. Review.
-
Streptococcus suis Serotype 2 Infection Causes Host Immunomodulation through Induction of Thymic Atrophy.Infect Immun. 2020 Mar 23;88(4):e00950-19. doi: 10.1128/IAI.00950-19. Print 2020 Mar 23. Infect Immun. 2020. PMID: 31932328 Free PMC article.
-
Modulating cholesterol-rich lipid rafts to disrupt influenza A virus infection.Front Immunol. 2022 Sep 13;13:982264. doi: 10.3389/fimmu.2022.982264. eCollection 2022. Front Immunol. 2022. PMID: 36177026 Free PMC article. Review.
-
The thymus road to a T cell: migration, selection, and atrophy.Front Immunol. 2024 Aug 27;15:1443910. doi: 10.3389/fimmu.2024.1443910. eCollection 2024. Front Immunol. 2024. PMID: 39257583 Free PMC article. Review.
References
-
- 1Centers for Disease Control and Prevention (CDC). Swine influenza A (H1N1) infection in two children–Southern California March-April 2009. MMWR Morb Mortal Wkly Rep 2009; 58: 400–402. - PubMed
-
- 2Chowell G, Bertozzi SM, Colchero MA, Lopez-Gatell H, Alpuche-Aranda C, Hernandez M et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med 2009; 361: 674–679. - PubMed
-
- 3Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA 2009; 302: 1872–1879. - PubMed
-
- 4Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quinones-Falconi F, Bautista E et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med 2009; 361: 680–689. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

