Abeta(1-42) enhances neuronal excitability in the CA1 via NR2B subunit-containing NMDA receptors
- PMID: 25276438
- PMCID: PMC4168240
- DOI: 10.1155/2014/584314
Abeta(1-42) enhances neuronal excitability in the CA1 via NR2B subunit-containing NMDA receptors
Abstract
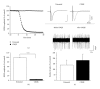
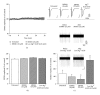
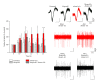
Neuronal hyperexcitability is a phenomenon associated with early Alzheimer's disease. The underlying mechanism is considered to involve excessive activation of glutamate receptors; however, the exact molecular pathway remains to be determined. Extracellular recording from the CA1 of hippocampal slices is a long-standing standard for a range of studies both in basic research and in neuropharmacology. Evoked field potentials (fEPSPs) are regarded as the input, while spiking rate is regarded as the output of the neuronal network; however, the relationship between these two phenomena is not fully clear. We investigated the relationship between spontaneous spiking and evoked fEPSPs using mouse hippocampal slices. Blocking AMPA receptors (AMPARs) with CNQX abolished fEPSPs, but left firing rate unchanged. NMDA receptor (NMDAR) blockade with MK801 decreased neuronal spiking dose dependently without altering fEPSPs. Activating NMDARs by small concentration of NMDA induced a trend of increased firing. These results suggest that fEPSPs are mediated by synaptic activation of AMPARs, while spontaneous firing is regulated by the activation of extrasynaptic NMDARs. Synaptotoxic Abeta(1-42) increased firing activity without modifying evoked fEPSPs. This hyperexcitation was prevented by ifenprodil, an antagonist of the NR2B NMDARs. Overall, these results suggest that Abeta(1-42) induced neuronal overactivity is not dependent on AMPARs but requires NR2B.
Figures



References
-
- Dingledine R, Dodd J, Kelly JS. The in vitro brain slice as a useful neurophysiological preparation for intracellular recording. Journal of Neuroscience Methods. 1980;2(4):323–362. - PubMed
-
- Tominaga T, Tominaga Y, Ichikawa M. Optical imaging of long-lasting depolarization on burst stimulation in area CA1 of rat hippocampal slices. Journal of Neurophysiology. 2002;88(3):1523–1532. - PubMed
-
- Diamond JS, Bergles DE, Jahr CE. Glutamate release monitored with astrocyte transporter currents during LTP. Neuron. 1998;21(2):425–433. - PubMed
-
- Lüscher C, Malenka RC, Nicoll RA. Monitoring glutamate release during LTP with glial transporter currents. Neuron. 1998;21(2):435–441. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

