Enantioselective hydroacylation of olefins with rhodium catalysts
- PMID: 25277153
- PMCID: PMC4456096
- DOI: 10.1039/c4cc02276a
Enantioselective hydroacylation of olefins with rhodium catalysts
Abstract
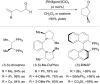
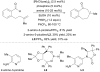
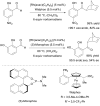
Over thirty years ago, James and Young reported the first enantioselective olefin hydroacylation by using rhodium catalysts. This viewpoint highlights the advances in this area, including 4-pentenal cyclisations, medium-ring syntheses, and intermolecular variants.
Figures
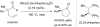

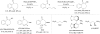
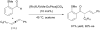
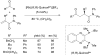
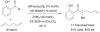


References
-
- Sakai K, Ide J, Oda O, Nakamura N. Tetrahedron Lett. 1972;13:1287.
-
-
For a comprehensive review on hydroacylation up to 2009, see Willis MC. Chem Rev. 2010;110:715..
-
-
- James BR, Young CG. J Chem Soc, Chem Commun. 1983:1215.
- James BR, Young CG. J Organomet Chem. 1985;285:321.
-
- Fairlie DP, Bosnich B. Organometallics. 1988;7:936.
- Fairlie DP, Bosnich B. Organometallics. 1988;7:946.
-
- Wu XM, Funakoshi K, Sakai K. Tetrahedron Lett. 1992;33:6331.
- Barnhart RW, Wang X, Noheda P, Bergens SH, Whelan J, Bosnich B. J Am Chem Soc. 1994;116:1821.
- Barnhart RW, McMorran DA, Bosnich B. Chem Commun. 1997:589.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

