Strategies for modern biomarker and drug development in oncology
- PMID: 25277503
- PMCID: PMC4189730
- DOI: 10.1186/s13045-014-0070-8
Strategies for modern biomarker and drug development in oncology
Abstract
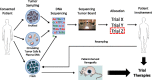
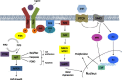
Technological advancements in the molecular characterization of cancers have enabled researchers to identify an increasing number of key molecular drivers of cancer progression. These discoveries have led to multiple novel anticancer therapeutics, and clinical benefit in selected patient populations. Despite this, the identification of clinically relevant predictive biomarkers of response continues to lag behind. In this review, we discuss strategies for the molecular characterization of cancers and the importance of biomarkers for the development of novel antitumor therapeutics. We also review critical successes and failures in oncology, and detail the lessons learnt, which may aid in the acceleration of anticancer drug development and biomarker discovery.
Figures
References
-
- Subbiah V, Westin SN, Wang K, Araujo D, Wang WL, Miller VA, Ross JS, Stephens PJ, Palmer GA, Ali SM. Targeted therapy by combined inhibition of the RAF and mTOR kinases in malignant spindle cell neoplasm harboring the KIAA1549-BRAF fusion protein. J Hematol Oncol. 2014;7(1):8. doi: 10.1186/1756-8722-7-8. - DOI - PMC - PubMed
-
- Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, Ou SH, Dezube BJ, Janne PA, Costa DB, Varella-Garcia M, Kim WH, Lynch TJ, Fidias P, Stubbs H, Engelman JA, Sequist LV, Tan W, Gandhi L, Mino-Kenudson M, Wei GC, Shreeve SM, Ratain MJ, Settleman J, Christensen JG, Haber DA, Wilner K, Salgia R, Shapiro GI, Clark JW. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–1703. doi: 10.1056/NEJMoa1006448. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

