Mechanisms of integral membrane protein insertion and folding
- PMID: 25277655
- PMCID: PMC4339636
- DOI: 10.1016/j.jmb.2014.09.014
Mechanisms of integral membrane protein insertion and folding
Abstract
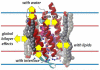
The biogenesis, folding, and structure of α-helical membrane proteins (MPs) are important to understand because they underlie virtually all physiological processes in cells including key metabolic pathways, such as the respiratory chain and the photosystems, as well as the transport of solutes and signals across membranes. Nearly all MPs require translocons--often referred to as protein-conducting channels--for proper insertion into their target membrane. Remarkable progress toward understanding the structure and functioning of translocons has been made during the past decade. Here, we review and assess this progress critically. All available evidence indicates that MPs are equilibrium structures that achieve their final structural states by folding along thermodynamically controlled pathways. The main challenge for cells is the targeting and membrane insertion of highly hydrophobic amino acid sequences. Targeting and insertion are managed in cells principally by interactions between ribosomes and membrane-embedded translocons. Our review examines the biophysical and biological boundaries of MP insertion and the folding of polytopic MPs in vivo. A theme of the review is the under-appreciated role of basic thermodynamic principles in MP folding and assembly. Thermodynamics not only dictates the final folded structure but also is the driving force for the evolution of the ribosome-translocon system of assembly. We conclude the review with a perspective suggesting a new view of translocon-guided MP insertion.
Keywords: lipid–protein interactions; membrane protein biogenesis; membrane protein folding; transmembrane helix.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures





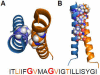

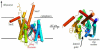



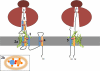
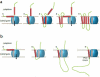
References
-
- White SH, Wimley WC. Membrane protein folding and stability: Physical principles. Annu Rev Biophys Biomol Struc. 1999;28:319–365. - PubMed
-
- Popot JL, Engelman DM. Helical membrane protein folding, stability, and evolution. Annu Rev Biochem. 2000;69:881–922. - PubMed
-
- MacKenzie KR. Folding and stability of a-helical integral membrane proteins. Chemical Reviews. 2006;106:1931–1977. - PubMed
-
- Goldenberg DP. Mutational analysis of protein folding and stability. In: Creighton TE, editor. Protein Folding. W. H. Freeman; New York: 1992. pp. 353–403.
-
- Yang AS, Sharp KA, Honig B. Analysis of the heat capacity dependence of protein folding. JMolBiol. 1992;227:889–900. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

