Evidence of effective delivery of the human papillomavirus (HPV) vaccine through a publicly funded, school-based program: the Ontario Grade 8 HPV Vaccine Cohort Study
- PMID: 25278003
- PMCID: PMC4198791
- DOI: 10.1186/1471-2458-14-1029
Evidence of effective delivery of the human papillomavirus (HPV) vaccine through a publicly funded, school-based program: the Ontario Grade 8 HPV Vaccine Cohort Study
Abstract
Background: Proper administration of the human papillomavirus (HPV) vaccine (three doses at 0, 2, and 6 months) will likely influence the vaccine's effectiveness and the impact of vaccination programs on health outcomes. Therefore, we assessed HPV vaccine series completion and on-time dosing in Canada's largest publicly funded, school-based HPV vaccination program.
Methods: Using administrative health and immunization databases, we identified a population-based cohort of girls eligible for Ontario's Grade 8 HPV vaccination program in the 2007/08-2009/10 program years who received at least one dose of the vaccine. We determined the number of doses received and calculated the percentage of girls that completed the three-dose series in Grade 8 and Grades 8-9. To assess on-time dosing, the number of days between doses 1-2, 2-3, and 1-3 was calculated and categorized (e.g., too short, on schedule, too long) based on the manufacturer's recommendations. Analyses were also stratified by program year.
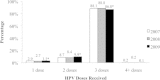
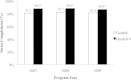
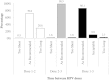
Results: We identified a cohort of 55,798 girls who initiated the vaccination series. Series completion was high in the Grade 8 window (81.8%) and increased by approximately 6% in Grade 9. Series completion was similar across the three program years. 70.8%, 98.5%, and 86.1% of girls were classified as 'on schedule' for dosing intervals 1-2, 2-3, and 1-3, respectively; 70.0% of girls received all three doses in perfect accordance with dosing recommendations. Stratification revealed that on-time dosing was highest in the first two years of the program (85.6% and 80.6%), but dropped to 42.1% in the 2009/10 year when H1N1 vaccination programs were prioritized.
Conclusions: Our study demonstrates that delivery of the HPV vaccine through a free, school-based program is an effective method of ensuring high completion and on-time dosing, but may not be sufficient to guarantee high coverage.
Figures
Similar articles
-
Factors associated with initiation and completion of the quadrivalent human papillomavirus vaccine series in an Ontario cohort of grade 8 girls.BMC Public Health. 2011 Aug 13;11:645. doi: 10.1186/1471-2458-11-645. BMC Public Health. 2011. PMID: 21838921 Free PMC article.
-
Coverage from Ontario, Canada's school-based HPV vaccine program: the first three years.Vaccine. 2013 Jan 21;31(5):757-62. doi: 10.1016/j.vaccine.2012.11.090. Epub 2012 Dec 13. Vaccine. 2013. PMID: 23246265
-
Implementation of a human papillomavirus vaccination demonstration project in Malawi: successes and challenges.BMC Public Health. 2017 Jun 26;17(1):599. doi: 10.1186/s12889-017-4526-y. BMC Public Health. 2017. PMID: 28651574 Free PMC article.
-
Literature review of HPV vaccine delivery strategies: considerations for school- and non-school based immunization program.Vaccine. 2014 Jan 9;32(3):320-6. doi: 10.1016/j.vaccine.2013.11.070. Epub 2013 Dec 2. Vaccine. 2014. PMID: 24295804 Review.
-
The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: what additional disease burden will a nonavalent vaccine prevent?Euro Surveill. 2018 Oct;23(41):1700737. doi: 10.2807/1560-7917.ES.2018.23.41.1700737. Euro Surveill. 2018. PMID: 30326995 Free PMC article. Review.
Cited by
-
Improving adolescent human papillomavirus (HPV) immunization uptake in school-based health centers through awareness campaigns.Vaccine. 2021 Mar 19;39(12):1765-1772. doi: 10.1016/j.vaccine.2021.02.006. Epub 2021 Feb 25. Vaccine. 2021. PMID: 33640146 Free PMC article.
-
Individual- and regional-level determinants of human papillomavirus (HPV) vaccine refusal: the Ontario Grade 8 HPV vaccine cohort study.BMC Public Health. 2014 Oct 8;14:1047. doi: 10.1186/1471-2458-14-1047. BMC Public Health. 2014. PMID: 25297055 Free PMC article.
-
Women's Knowledge of Cervical Cancer: A cross-sectional study in Al Buraimi Governorate, Oman.Sultan Qaboos Univ Med J. 2021 Aug;21(3):450-456. doi: 10.18295/squmj.4.2021.022. Epub 2021 Aug 29. Sultan Qaboos Univ Med J. 2021. PMID: 34522412 Free PMC article.
-
Parents' and providers' attitudes toward school-located provision and school-entry requirements for HPV vaccines.Hum Vaccin Immunother. 2016 Jun 2;12(6):1606-14. doi: 10.1080/21645515.2016.1140289. Epub 2016 Mar 2. Hum Vaccin Immunother. 2016. PMID: 26934421 Free PMC article.
-
Highest Vaccine Uptake after School-Based Delivery - A County-Level Evaluation of the Implementation Strategies for HPV Catch-Up Vaccination in Sweden.PLoS One. 2016 Mar 14;11(3):e0149857. doi: 10.1371/journal.pone.0149857. eCollection 2016. PLoS One. 2016. PMID: 26974977 Free PMC article. Clinical Trial.
References
-
- Merck Canada Inc . Product Monograph - Gardasil® [Quadrivalent human papillomavirus (types 6, 11, 16, 18) Recombinant Vaccine] 2013.
Pre-publication history
-
- The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2458/14/1029/prepub
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

