The E3 ubiquitin ligase MID1 catalyzes ubiquitination and cleavage of Fu
- PMID: 25278022
- PMCID: PMC4231658
- DOI: 10.1074/jbc.M113.541219
The E3 ubiquitin ligase MID1 catalyzes ubiquitination and cleavage of Fu
Abstract
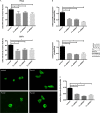
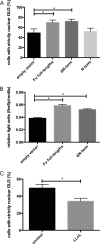
SHH (Sonic Hedgehog)-GLI signaling plays an important role during embryogenesis and in tumorigenesis. The survival and growth of several types of cancer depend on autonomously activated SHH-GLI signaling. A protein complex containing the ubiquitin ligase MID1 and protein phosphatase 2A regulates the nuclear localization and transcriptional activity of GLI3, a transcriptional effector molecule of SHH, in cancer cell lines with autonomously activated SHH signaling. However, the exact molecular mechanisms that mediate the interaction between MID1 and GLI3 remained unknown. Here, we show that MID1 catalyzes the ubiquitination and proteasomal cleavage of the GLI3 regulator Fu. Our data suggest that Fu ubiquitination and cleavage is one of the key elements connecting the MID1-PP2A protein complex with GLI3 activity control.
Keywords: Cancer Biology; Fu; GLI3; MID1; Proteasomal Cleavage; Proteasome; Protein Phosphatase 2 (PP2A); Signal transduction; Ubiquitination.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Nybakken K., Perrimon N. (2002) Hedgehog signal transduction: recent findings. Curr. Opin. Genet. Dev. 12, 503–511 - PubMed
-
- Ingham P. W., McMahon A. P. (2001) Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 15, 3059–3087 - PubMed
-
- Murone M., Rosenthal A., de Sauvage F. J. (1999) Hedgehog signal transduction: from flies to vertebrates. Exp. Cell Res. 253, 25–33 - PubMed
-
- Nybakken K. E., Turck C. W., Robbins D. J., Bishop J. M. (2002) Hedgehog-stimulated phosphorylation of the kinesin-related protein Costal2 is mediated by the serine/threonine kinase fused. J. Biol. Chem. 277, 24638–24647 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

