Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study
- PMID: 25278221
- PMCID: PMC7106357
- DOI: 10.1016/S1473-3099(14)70920-X
Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study
Erratum in
- Lancet Infect Dis. 2015 Jan 15;211(2):13
Abstract
Background: Middle East respiratory syndrome coronavirus (MERS-CoV) infection is associated with high mortality and has no approved antiviral therapy. We aimed to compare ribavirin and interferon alfa-2a treatment for patients with severe MERS-CoV infection with a supportive therapy only.
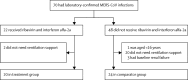
Methods: In this retrospective cohort study, we included adults (aged ≥16 years) with laboratory-confirmed MERS-CoV infection and pneumonia needing ventilation support, diagnosed between Oct 23, 2012, and May 1, 2014, at the Prince Sultan Military Medical City (Riyadh, Saudi Arabia). All patients received appropriate supportive care and regular clinical and laboratory monitoring, but patients diagnosed after Sept 16, 2013, were also given oral ribavirin (dose based on calculated creatinine clearance, for 8-10 days) and subcutaneous pegylated interferon alfa-2a (180 μg per week for 2 weeks). The primary endpoint was 14-day and 28-day survival from the date of MERS-CoV infection diagnosis. We used χ(2) and Fischer's exact test to analyse categorical variables and the t test to analyse continuous variables.
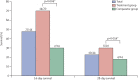
Findings: We analysed 20 patients who received ribavirin and interferon (treatment group; initiated a median of 3 days [range 0-8] after diagnosis) and 24 who did not (comparator group). Baseline clinical and laboratory characteristics were similar between groups, apart from baseline absolute neutrophil count, which was significantly lower in the comparator group (5·88 × 10(9)/L [SD 3·95] vs 9·88 × 10(9)/L [6·63]; p=0·023). 14 (70%) of 20 patients in the treatment group had survived after 14 days, compared with seven (29%) of 24 in the comparator group (p=0·004). After 28 days, six (30%) of 20 and four (17%) of 24, respectively, had survived (p=0·54). Adverse effects were similar between groups, apart from reduction in haemoglobin, which was significantly greater in the treatment group than in the comparator group (4·32 g/L [SD 2·47] vs 2·14 g/L [1·90]; p=0·002).
Interpretation: In patients with severe MERS-CoV infection, ribavirin and interferon alfa-2a therapy is associated with significantly improved survival at 14 days, but not at 28 days. Further assessment in appropriately designed randomised trials is recommended.
Funding: None.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Treating MERS-CoV during an outbreak.Lancet Infect Dis. 2014 Nov;14(11):1030-1031. doi: 10.1016/S1473-3099(14)70939-9. Epub 2014 Sep 29. Lancet Infect Dis. 2014. PMID: 25278219 Free PMC article. No abstract available.
References
-
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus ADME, Fouchier RAM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. - PubMed
-
- European Centre for Disease Prevention and Control Severe respiratory disease associated with Middle East respiratory syndrome coronavirus (MERS-CoV)–11th update. August 21, 2014. http://www.ecdc.europa.eu/en/publications/Publications/Middle-East-respi... (accessed Sept 17, 2014).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources