Sepsis lethality via exacerbated tissue infiltration and TLR-induced cytokine production by neutrophils is integrin α3β1-dependent
- PMID: 25278585
- PMCID: PMC4256905
- DOI: 10.1182/blood-2014-01-552943
Sepsis lethality via exacerbated tissue infiltration and TLR-induced cytokine production by neutrophils is integrin α3β1-dependent
Abstract
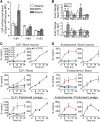
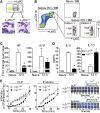
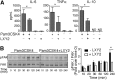
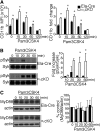
Integrin-mediated migration of neutrophils to infected tissue sites is vital for pathogen clearance and therefore host survival. Although β2 integrins have been shown to mediate neutrophil transendothelial migration during systemic and local inflammation, relatively little information is available regarding neutrophil migration in sepsis beyond the endothelial cell layer. In this study, we report that integrin α3β1 (VLA-3; CD49c/CD29) is dramatically upregulated on neutrophils isolated from both human septic patients and in mouse models of sepsis. Compared with the α3β1 (low) granulocytes, α3β1 (high) cells from septic animals displayed hyperinflammatory phenotypes. Administration of a α3β1 blocking peptide and conditional deletion of α3 in granulocytes significantly reduced the number of extravasating neutrophils and improved survival in septic mice. In addition, expression of α3β1 on neutrophils was associated with Toll-like receptor-induced inflammatory responses and cytokine productions. Thus, our results show that α3β1 is a novel marker of tissue homing and hyperresponsive neutrophil subtypes in sepsis, and blocking of α3β1 may represent a new therapeutic approach in sepsis treatment.
© 2014 by The American Society of Hematology.
Figures



Comment in
-
α3β1 is INTEGRAL to septic neutrophils.Blood. 2014 Dec 4;124(24):3507-8. doi: 10.1182/blood-2014-10-606632. Blood. 2014. PMID: 25477480 No abstract available.
References
-
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. - PubMed
-
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6(3):173–182. - PubMed
-
- Brown KA, Brain SD, Pearson JD, Edgeworth JD, Lewis SM, Treacher DF. Neutrophils in development of multiple organ failure in sepsis. Lancet. 2006;368(9530):157–169. - PubMed
-
- Hogg N, Patzak I, Willenbrock F. The insider’s guide to leukocyte integrin signalling and function. Nat Rev Immunol. 2011;11(6):416–426. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

