A synaptic mechanism for network synchrony
- PMID: 25278839
- PMCID: PMC4166887
- DOI: 10.3389/fncel.2014.00290
A synaptic mechanism for network synchrony
Abstract
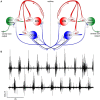
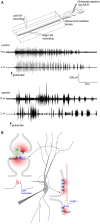
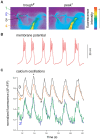
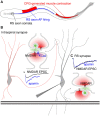
Within neural networks, synchronization of activity is dependent upon the synaptic connectivity of embedded microcircuits and the intrinsic membrane properties of their constituent neurons. Synaptic integration, dendritic Ca(2+) signaling, and non-linear interactions are crucial cellular attributes that dictate single neuron computation, but their roles promoting synchrony and the generation of network oscillations are not well understood, especially within the context of a defined behavior. In this regard, the lamprey spinal central pattern generator (CPG) stands out as a well-characterized, conserved vertebrate model of a neural network (Smith et al., 2013a), which produces synchronized oscillations in which neural elements from the systems to cellular level that control rhythmic locomotion have been determined. We review the current evidence for the synaptic basis of oscillation generation with a particular emphasis on the linkage between synaptic communication and its cellular coupling to membrane processes that control oscillatory behavior of neurons within the locomotor network. We seek to relate dendritic function found in many vertebrate systems to the accessible lamprey central nervous system in which the relationship between neural network activity and behavior is well understood. This enables us to address how Ca(2+) signaling in spinal neuron dendrites orchestrate oscillations that drive network behavior.
Keywords: KCa2; NMDA; SK2; calcium; dendrites; lamprey; locomotion; oscillation.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

