Outcome of central nervous system relapses in childhood acute lymphoblastic leukaemia--prospective open cohort analyses of the ALLR3 trial
- PMID: 25279465
- PMCID: PMC4184796
- DOI: 10.1371/journal.pone.0108107
Outcome of central nervous system relapses in childhood acute lymphoblastic leukaemia--prospective open cohort analyses of the ALLR3 trial
Abstract
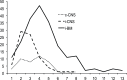
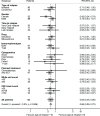
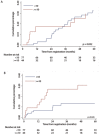
The outcomes of Central Nervous System (CNS) relapses in children with acute lymphoblastic leukaemia (ALL) treated in the ALL R3 trial, between January 2003 and March 2011 were analysed. Patients were risk stratified, to receive a matched donor allogeneic transplant or fractionated cranial irradiation with continued treatment for two years. A randomisation of Idarubicin with Mitoxantrone closed in December 2007 in favour of Mitoxantrone. The estimated 3-year progression free survival for combined and isolated CNS disease were 40.6% (25·1, 55·6) and 38.0% (26.2, 49.7) respectively. Univariate analysis showed a significantly better survival for age <10 years, progenitor-B cell disease, good-risk cytogenetics and those receiving Mitoxantrone. Adjusting for these variables (age, time to relapse, cytogenetics, treatment drug and gender) a multivariate analysis, showed a poorer outcome for those with combined CNS relapse (HR 2·64, 95% CI 1·32, 5·31, p = 0·006 for OS). ALL R3 showed an improvement in outcome for CNS relapses treated with Mitoxantrone compared to Idarubicin; a potential benefit for matched donor transplant for those with very early and early isolated-CNS relapses.
Trial registration: Controlled-Trials.com ISRCTN45724312.
Conflict of interest statement
Figures

References
-
- Conter V, Arico M, Basso G, Biondi A, Barisone E, et al. (2010) Long-term results of the Italian Association of Pediatric Hematology and Oncology (AIEOP) Studies 82, 87, 88, 91 and 95 for childhood acute lymphoblastic leukemia. Leukemia 24: 255–264. - PubMed
-
- Kamps WA, van der Pal-de Bruin KM, Veerman AJ, Fiocco M, Bierings M, et al. (2010) Long-term results of Dutch Childhood Oncology Group studies for children with acute lymphoblastic leukemia from 1984 to 2004. Leukemia 24: 309–319. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

