High expression of hTERT and stemness genes in BORIS/CTCFL positive cells isolated from embryonic cancer cells
- PMID: 25279549
- PMCID: PMC4184884
- DOI: 10.1371/journal.pone.0109921
High expression of hTERT and stemness genes in BORIS/CTCFL positive cells isolated from embryonic cancer cells
Abstract
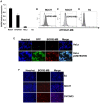
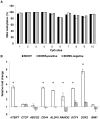
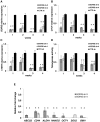
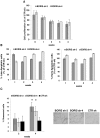
BORIS/CTCFL is a member of cancer testis antigen family normally expressed in germ cells. In tumors, it is aberrantly expressed although its functions are not completely well-defined. To better understand the functions of BORIS in cancer, we selected the embryonic cancer cells as a model. Using a molecular beacon, which specifically targets BORIS mRNA, we demonstrated that BORIS positive cells are a small subpopulation of tumor cells (3-5% of total). The BORIS-positive cells isolated using BORIS-molecular beacon, expressed higher telomerase hTERT, stem cell (NANOG, OCT4, SOX2) and cancer stem cell marker genes (CD44 and ALDH1) compared to the BORIS-negative tumor cells. In order to define the functional role of BORIS, stable BORIS-depleted embryonic cancer cells were generated. BORIS silencing strongly down-regulated the expression of hTERT, stem cell and cancer stem cell marker genes. Moreover, the BORIS knockdown increased cellular senescence in embryonic cancer cells, revealing a putative role of BORIS in the senescence biological program. Our data indicate an association of BORIS expressing cells subpopulation with the expression of stemness genes, highlighting the critical role played by BORIS in embryonic neoplastic disease.
Conflict of interest statement
Figures


References
-
- Dunn KL, Davie JR (2003) The many roles of the transcriptional regulator CTCF. Biochem Cell Biol 81: 161–167. - PubMed
-
- Herold M, Bartkuhn M, Renkawitz R (2012) CTCF: insights into insulator function during development. Development 139: 1045–1057. - PubMed
-
- Loukinov DI, Pugacheva E, Vatolin S, Pack SD, Moon H, et al. (2002) BORIS, a novel male germ-line-specific protein associated with epigenetic reprogramming events, shares the same 11-zinc-finger domain with CTCF, the insulator protein involved in reading imprinting marks in the soma. Proc Natl Acad Sci USA 99: 6806–6811. - PMC - PubMed
-
- Klenova EM, Morse HC 3rd, Ohlsson R, Lobanenkov VV (2002) The novel BORIS + CTCF gene family is uniquely involved in the epigenetics of normal biology and cancer. Semin Cancer Biol 12: 399–414. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

