Biomarker correlates of survival in pediatric patients with Ebola virus disease
- PMID: 25279581
- PMCID: PMC4193175
- DOI: 10.3201/eid2010.140430
Biomarker correlates of survival in pediatric patients with Ebola virus disease
Abstract
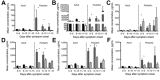
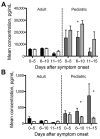
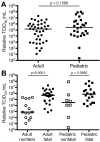
Outbreaks of Ebola virus disease (EVD) occur sporadically in Africa and are associated with high case-fatality rates. Historically, children have been less affected than adults. The 2000-2001 Sudan virus-associated EVD outbreak in the Gulu district of Uganda resulted in 55 pediatric and 161 adult laboratory-confirmed cases. We used a series of multiplex assays to measure the concentrations of 55 serum analytes in specimens from patients from that outbreak to identify biomarkers specific to pediatric disease. Pediatric patients who survived had higher levels of the chemokine regulated on activation, normal T-cell expressed and secreted marker and lower levels of plasminogen activator inhibitor 1, soluble intracellular adhesion molecule, and soluble vascular cell adhesion molecule than did pediatric patients who died. Adult patients had similar levels of these analytes regardless of outcome. Our findings suggest that children with EVD may benefit from different treatment regimens than those for adults.
Figures
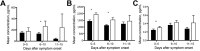
References
-
- Fields BN, Knipe DM, Howley PM, editors. Fields’ virology. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2007.
-
- Centers for Disease Control and Prevention. Outbreak of Ebola hemorrhagic fever, Uganda, August 2000. –January 2001. 2001 Feb 9. Report nos. 0149–2195/0149–2195.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

