Understanding cadherin EGF LAG seven-pass G-type receptors
- PMID: 25280249
- PMCID: PMC4261025
- DOI: 10.1111/jnc.12955
Understanding cadherin EGF LAG seven-pass G-type receptors
Abstract
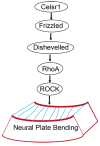
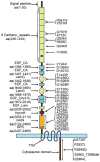
The cadherin epidermal growth factor (EGF) laminin G (LAG) seven-pass G-type receptors (CELSRs) are a special subgroup of adhesion G protein-coupled receptors, which are pivotal regulators of many biologic processes such as neuronal/endocrine cell differentiation, vessel valve formation, and the control of planar cell polarity during embryonic development. All three members of the CELSR family (CELSR1-3) have large ecto-domains that form homophilic interactions and encompass more than 2000 amino acids. Mutations in the ecto-domain or other gene locations of CELSRs are associated with neural tube defects and other diseases in humans. Celsr knockout (KO) animals have many developmental defects. Therefore, specific agonists or antagonists of CELSR members may have therapeutic potential. Although significant progress has been made regarding the functions and biochemical properties of CELSRs, our knowledge of these receptors is still lacking, especially considering that they are broadly distributed but have few characterized functions in a limited number of tissues. The dynamic activation and inactivation of CELSRs and the presence of endogenous ligands beyond homophilic interactions remain elusive, as do the regulatory mechanisms and downstream signaling of these receptors. Given this motivation, future studies with more advanced cell biology or biochemical tools, such as conditional KO mice, may provide further insights into the mechanisms underlying CELSR function, laying the foundation for the design of new CELSR-targeted therapeutic reagents. The cadherin EGF LAG seven-pass G-type receptors (CELSRs) are a special subgroup of adhesion G protein-coupled receptors (GPCRs), which have large ecto-domains that form homophilic interactions and encompass more than 2000 amino acids. Recent studies have revealed that CELSRs are pivotal regulators of many biological processes, such as neuronal/endocrine cell differentiation, vessel valve formation and the control of planar cell polarity during embryonic development.
Keywords: CELSR; G protein-coupled receptor; adhesion; development; planar cell polarity.
© 2014 International Society for Neurochemistry.
Figures


References
-
- Allache R, De Marco P, Merello E, Capra V, Kibar Z. Role of the planar cell polarity gene CELSR1 in neural tube defects and caudal agenesis. Birth Defects Res A Clin Mol Teratol. 2012;94:176–181. - PubMed
-
- Ammerpohl O, Pratschke J, Schafmayer C, et al. Distinct DNA methylation patterns in cirrhotic liver and hepatocellular carcinoma. Int J Cancer. 2012;130:1319–1328. - PubMed
-
- Arvind P, Nair J, Jambunathan S, Kakkar VV, Shanker J. CELSR2-PSRC1-SORT1 gene expression and association with coronary artery disease and plasma lipid levels in an Asian Indian cohort. J Cardiol 2014 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

