Ceramide-mediated depression in cardiomyocyte contractility through PKC activation and modulation of myofilament protein phosphorylation
- PMID: 25280528
- PMCID: PMC4440670
- DOI: 10.1007/s00395-014-0445-6
Ceramide-mediated depression in cardiomyocyte contractility through PKC activation and modulation of myofilament protein phosphorylation
Abstract
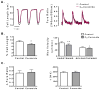
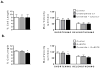
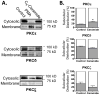
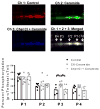
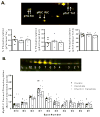
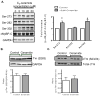
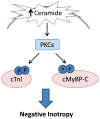
Although ceramide accumulation in the heart is considered a major factor in promoting apoptosis and cardiac disorders, including heart failure, lipotoxicity and ischemia-reperfusion injury, little is known about ceramide's role in mediating changes in contractility. In the present study, we measured the functional consequences of acute exposure of isolated field-stimulated adult rat cardiomyocytes to C6-ceramide. Exogenous ceramide treatment depressed the peak amplitude and the maximal velocity of shortening without altering intracellular calcium levels or kinetics. The inactive ceramide analog C6-dihydroceramide had no effect on myocyte shortening or [Ca(2+)]i transients. Experiments testing a potential role for C6-ceramide-mediated effects on activation of protein kinase C (PKC) demonstrated evidence for signaling through the calcium-independent isoform, PKCε. We employed 2-dimensional electrophoresis and anti-phospho-peptide antibodies to test whether treatment of the cardiomyocytes with C6-ceramide altered myocyte shortening via PKC-dependent phosphorylation of myofilament proteins. Compared to controls, myocytes treated with ceramide exhibited increased phosphorylation of myosin binding protein-C (cMyBP-C), specifically at Ser273 and Ser302, and troponin I (cTnI) at sites apart from Ser23/24, which could be attenuated with PKC inhibition. We conclude that the altered myofilament response to calcium resulting from multiple sites of PKC-dependent phosphorylation contributes to contractile dysfunction that is associated with cardiac diseases in which elevations in ceramides are present.
Conflict of interest statement
None
Figures



References
-
- Ayaz-Guner S, Zhang J, Li L, Walker JW, Ge Y. In vivo phosphorylation site mapping in mouse cardiac troponin I by high resolution top-down electron capture dissociation mass spectrometry: Ser22/23 are the only sites basally phosphorylated. Biochemistry. 2009;48:8161–8170. doi: 10.1021/bi900739f. - DOI - PMC - PubMed
-
- Bielawska A, Crane HM, Liotta D, Obeid LM, Hannun YA. Selectivity of ceramide-mediated biology. Lack of activity of erythro-dihydroceramide. J Biol Chem. 1993;268:26226–26232. - PubMed
-
- Brutsaert DL, Sys SU. Relaxation and diastole of the heart. Physiol Rev. 1989;69:1228–1315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

