Roles of TBC1D1 and TBC1D4 in insulin- and exercise-stimulated glucose transport of skeletal muscle
- PMID: 25280670
- PMCID: PMC4258142
- DOI: 10.1007/s00125-014-3395-5
Roles of TBC1D1 and TBC1D4 in insulin- and exercise-stimulated glucose transport of skeletal muscle
Abstract
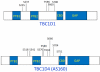
This review focuses on two paralogue Rab GTPase activating proteins known as TBC1D1 Tre-2/BUB2/cdc 1 domain family (TBC1D) 1 and TBC1D4 (also called Akt Substrate of 160 kDa, AS160) and their roles in controlling skeletal muscle glucose transport in response to the independent and combined effects of insulin and exercise. Convincing evidence implicates Akt2-dependent TBC1D4 phosphorylation on T642 as a key part of the mechanism for insulin-stimulated glucose uptake by skeletal muscle. TBC1D1 phosphorylation on several insulin-responsive sites (including T596, a site corresponding to T642 in TBC1D4) does not appear to be essential for in vivo insulin-stimulated glucose uptake by skeletal muscle. In vivo exercise or ex vivo contraction of muscle result in greater TBC1D1 phosphorylation on S237 that is likely to be secondary to increased AMP-activated protein kinase activity and potentially important for contraction-stimulated glucose uptake. Several studies that evaluated both normal and insulin-resistant skeletal muscle stimulated with a physiological insulin concentration after a single exercise session found that greater post-exercise insulin-stimulated glucose uptake was accompanied by greater TBC1D4 phosphorylation on several sites. In contrast, enhanced post-exercise insulin sensitivity was not accompanied by greater insulin-stimulated TBC1D1 phosphorylation. The mechanism for greater TBC1D4 phosphorylation in insulin-stimulated muscles after acute exercise is uncertain, and a causal link between enhanced TBC1D4 phosphorylation and increased post-exercise insulin sensitivity has yet to be established. In summary, TBC1D1 and TBC1D4 have important, but distinct roles in regulating muscle glucose transport in response to insulin and exercise.
Figures

References
-
- Constable SH, Favier RJ, Cartee GD, Young DA, Holloszy JO. Muscle glucose transport: interactions of in vitro contractions, insulin, and exercise. J Appl Physiol. 1988;64:2329–2332. - PubMed
-
- Cartee GD, Wojtaszewski JF. Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport. Appl Physiol Nutr Metab. 2007;32:557–566. - PubMed
-
- Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev. 2013;93:993–1017. - PubMed
-
- Douen AG, Ramlal T, Rastogi S, et al. Exercise induces recruitment of the “insulin-responsive glucose transporter”. Evidence for distinct intracellular insulin- and exercise-recruitable transporter pools in skeletal muscle. J Biol Chem. 1990;265:13427–13430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

