The neural basis of image segmentation in the primate brain
- PMID: 25280789
- PMCID: PMC4383733
- DOI: 10.1016/j.neuroscience.2014.09.051
The neural basis of image segmentation in the primate brain
Abstract
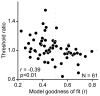
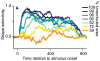
Image segmentation is a fundamental aspect of vision and a critical part of scene understanding. Our visual system rapidly and effortlessly segments scenes into component objects but the underlying neural basis is unknown. We studied single neurons in area V4 while monkeys discriminated partially occluded shapes. We found that many neurons tuned to boundary curvature maintained their shape selectivity over a large range of occlusion levels as compared to neurons that are not tuned to boundary curvature. This lends support to the hypothesis that segmentation in the face of occlusion may be solved by contour grouping.
Keywords: monkey; object recognition; shape representation; ventral pathway.
Copyright © 2014 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures




References
-
- Allen HA, Humphreys GW, Colin J, Neumann H. Ventral extra-striate cortical areas are required for human visual texture segmentation. J Vis. 2009;9(2):1–14. - PubMed
-
- Asaad WF, Rainer G, Miller EK. Neural activity in the primate prefrontal cortex during associative learning. Neuron. 1998;21:1399–407. - PubMed
-
- Ben-Av MB, Sagi D, Braun J. Visual attention and perceptual grouping. Percept Psychophys. 1992;52:277–294. - PubMed
-
- Berg DJ, Boehnke SE, Marino RA, Munoz DP, Itti L. Free viewing of dynamic stimuli by humans and monkeys. J Vis. 2009;9(19):1–15. - PubMed
-
- Bringuier V, Chavane F, Glaeser L, Fregnac Y. Horizontal propagation of visual activity in the synaptic integration field of area 17 neurons. Science. 1999;283:695–699. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

