A diagnostic approach for cerebral palsy in the genomic era
- PMID: 25280894
- PMCID: PMC4229412
- DOI: 10.1007/s12017-014-8331-9
A diagnostic approach for cerebral palsy in the genomic era
Abstract
An ongoing challenge in children presenting with motor delay/impairment early in life is to identify neurogenetic disorders with a clinical phenotype, which can be misdiagnosed as cerebral palsy (CP). To help distinguish patients in these two groups, conventional magnetic resonance imaging of the brain has been of great benefit in "unmasking" many of these genetic etiologies and has provided important clues to differential diagnosis in others. Recent advances in molecular genetics such as chromosomal microarray and next-generation sequencing have further revolutionized the understanding of etiology by more precisely classifying these disorders with a molecular cause. In this paper, we present a review of neurogenetic disorders masquerading as cerebral palsy evaluated at one institution. We have included representative case examples children presenting with dyskinetic, spastic, and ataxic phenotypes, with the intent to highlight the time-honored approach of using clinical tools of history and examination to focus the subsequent etiologic search with advanced neuroimaging modalities and molecular genetic tools. A precise diagnosis of these masqueraders and their differentiation from CP is important in terms of therapy, prognosis, and family counseling. In summary, this review serves as a continued call to remain vigilant for current and other to-be-discovered neurogenetic masqueraders of cerebral palsy, thereby optimizing care for patients and their families.
Conflict of interest statement
The corresponding author is a paid member of the drug monitoring committee for BlueBirdBio, Inc. There are no other conflicts of interest.
Figures

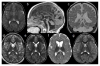
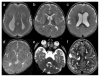
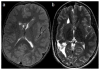
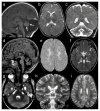
References
-
- Accardo PJCAJ. Capute & Accardo’s neurodevelopmental disabilities in infancy and childhood. Baltimore: Paul H. Brookes Pub; 2008.
-
- Ashwal S, Russman BS, Blasco PA, Miller G, Sandler A, Shevell M, et al. Practice parameter: diagnostic assessment of the child with cerebral palsy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2004;62(6):851–863. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

