Short intronic repeat sequences facilitate circular RNA production
- PMID: 25281217
- PMCID: PMC4201285
- DOI: 10.1101/gad.251926.114
Short intronic repeat sequences facilitate circular RNA production
Abstract
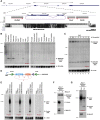
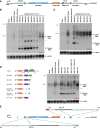
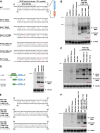
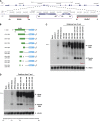
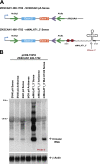
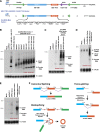
Recent deep sequencing studies have revealed thousands of circular noncoding RNAs generated from protein-coding genes. These RNAs are produced when the precursor messenger RNA (pre-mRNA) splicing machinery "backsplices" and covalently joins, for example, the two ends of a single exon. However, the mechanism by which the spliceosome selects only certain exons to circularize is largely unknown. Using extensive mutagenesis of expression plasmids, we show that miniature introns containing the splice sites along with short (∼ 30- to 40-nucleotide) inverted repeats, such as Alu elements, are sufficient to allow the intervening exons to circularize in cells. The intronic repeats must base-pair to one another, thereby bringing the splice sites into close proximity to each other. More than simple thermodynamics is clearly at play, however, as not all repeats support circularization, and increasing the stability of the hairpin between the repeats can sometimes inhibit circular RNA biogenesis. The intronic repeats and exonic sequences must collaborate with one another, and a functional 3' end processing signal is required, suggesting that circularization may occur post-transcriptionally. These results suggest detailed and generalizable models that explain how the splicing machinery determines whether to produce a circular noncoding RNA or a linear mRNA.
Keywords: Alu; EPHB4; HIPK3; ZKSCAN1; circRNA; noncoding RNA; splicing.
© 2014 Liang and Wilusz; Published by Cold Spring Harbor Laboratory Press.
Figures

References
-
- Begley DA, Berkenpas MB, Sampson KE, Abraham I. 1997. Identification and sequence of human PKY, a putative kinase with increased expression in multidrug-resistant cells, with homology to yeast protein kinase Yak1. Gene 200: 35–43 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
