Supporting breastfeeding In Local Communities (SILC): protocol for a cluster randomised controlled trial
- PMID: 25281300
- PMCID: PMC4287548
- DOI: 10.1186/1471-2393-14-346
Supporting breastfeeding In Local Communities (SILC): protocol for a cluster randomised controlled trial
Abstract
Background: Breastfeeding is associated with significant positive health outcomes for mothers and infants. However, despite recommendations from the World Health Organization, exclusive breastfeeding for six months is uncommon. Increased breastfeeding support early in the postpartum period may be effective in improving breastfeeding maintenance. This trial will evaluate two community-based interventions to increase breastfeeding duration in Local Government Areas (LGAs) in Victoria, Australia.
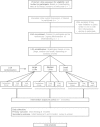
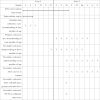
Methods/design: A three-arm cluster randomised controlled trial design will be used. Victorian LGAs with a lower than average rate of any breastfeeding at discharge from hospital and more than 450 births per year that agree to participate will be randomly allocated to one of three trial arms: 1) standard care; 2) home-based breastfeeding support; or 3) home-based breastfeeding support plus access to a community-based breastfeeding drop-in centre. The services provided in LGAs allocated to 'standard care' are those routinely available to postpartum women. LGAs allocated to the home-based visiting intervention will provide home-visits to women who are identified as at risk of breastfeeding cessation in the early postnatal period. These visits will be provided by Maternal and Child Health Nurses who have received training to provide the intervention (SILC-MCHNs). In areas allocated to receive the second intervention, in addition to home-based breastfeeding support, community breastfeeding drop-in centres will be made available, staffed by a SILC-MCHN. The interventions will run in LGAs for a nine to twelve month period depending on birth numbers. The primary outcome is the proportion of infants receiving any breast milk at four months of age. Breastfeeding outcomes will be obtained from routinely collected Maternal and Child Health centre data and from a new data item collecting infant feeding 'in the last 24 hours'. Information will also be obtained directly from women via a postal survey. A comprehensive process evaluation will be conducted.
Discussion: This study will determine if early home-based breastfeeding support by a health professional for women at risk of stopping breastfeeding, with or without access to a community-based breastfeeding drop-in centre, increases breastfeeding duration in Victorian LGAs with low breastfeeding rates.
Trial registration: Australian New Zealand Clinical Trials Registry: ACTRN12611000898954.
Figures
References
-
- Ip S, Chung M, Raman G, Chew P, Magula N, DeVine D, Trikalinos T, Lau J. Evidence Report/Technology Assessment No 153 (Prepared by Tufts-New England Medical Center Evidence-based Practice Center, under Contract No 290-02-0022) AHRQ Publication No 07-E007. Rockville, MD: Agency for Healthcare Research and Quality; 2007. Breastfeeding and Maternal and Infant Health Outcomes in Developed Countries. - PMC - PubMed
-
- Renfrew MJ, Pokhrel S, Quiqley M, McCormick F, Fox-Rushby J, Dodds R, Duffy S, Trueman P, Williams A. Preventing disease and saving resources: the potential contribution of increasing breastfeeding rates in the UK. London, UK: UNICEF; 2012.
Pre-publication history
-
- The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2393/14/346/prepub
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

