Intercellular adhesion molecule-1 expression by skeletal muscle cells augments myogenesis
- PMID: 25281303
- PMCID: PMC4323887
- DOI: 10.1016/j.yexcr.2014.09.032
Intercellular adhesion molecule-1 expression by skeletal muscle cells augments myogenesis
Abstract
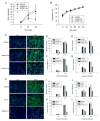
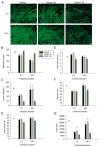
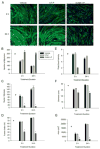
We previously demonstrated that the expression of intercellular adhesion molecule-1 (ICAM-1) by skeletal muscle cells after muscle overload contributes to ensuing regenerative and hypertrophic processes in skeletal muscle. The objective of the present study is to reveal mechanisms through which skeletal muscle cell expression of ICAM-1 augments regenerative and hypertrophic processes of myogenesis. This was accomplished by genetically engineering C2C12 myoblasts to stably express ICAM-1, and by inhibiting the adhesive and signaling functions of ICAM-1 through the use of a neutralizing antibody or cell penetrating peptide, respectively. Expression of ICAM-1 by cultured skeletal muscle cells augmented myoblast-myoblast adhesion, myotube formation, myonuclear number, myotube alignment, myotube-myotube fusion, and myotube size without influencing the ability of myoblasts to proliferate or differentiate. ICAM-1 augmented myotube formation, myonuclear accretion, and myotube alignment through a mechanism involving adhesion-induced activation of ICAM-1 signaling, as these dependent measures were reduced via antibody and peptide inhibition of ICAM-1. The adhesive and signaling functions of ICAM-1 also facilitated myotube hypertrophy through a mechanism involving myotube-myotube fusion, protein synthesis, and Akt/p70s6k signaling. Our findings demonstrate that ICAM-1 expression by skeletal muscle cells augments myogenesis, and establish a novel mechanism through which the inflammatory response facilitates growth processes in skeletal muscle.
Keywords: Adhesion molecules; Inflammation; Muscle hypertrophy; Muscle regeneration.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare there are no competing financial or non-financial interests.
Figures

Similar articles
-
A novel in vitro model for the assessment of postnatal myonuclear accretion.Skelet Muscle. 2018 Feb 14;8(1):4. doi: 10.1186/s13395-018-0151-4. Skelet Muscle. 2018. PMID: 29444710 Free PMC article.
-
Nilotinib impairs skeletal myogenesis by increasing myoblast proliferation.Skelet Muscle. 2018 Feb 20;8(1):5. doi: 10.1186/s13395-018-0150-5. Skelet Muscle. 2018. PMID: 29463296 Free PMC article.
-
Skeletal muscle cells express ICAM-1 after muscle overload and ICAM-1 contributes to the ensuing hypertrophic response.PLoS One. 2013;8(3):e58486. doi: 10.1371/journal.pone.0058486. Epub 2013 Mar 11. PLoS One. 2013. PMID: 23505517 Free PMC article.
-
Mechanisms regulating myoblast fusion: A multilevel interplay.Semin Cell Dev Biol. 2020 Aug;104:81-92. doi: 10.1016/j.semcdb.2020.02.004. Epub 2020 Feb 13. Semin Cell Dev Biol. 2020. PMID: 32063453 Review.
-
Galectin-1 is a novel factor that regulates myotube growth in regenerating skeletal muscles.Curr Drug Targets. 2005 Jun;6(4):395-405. doi: 10.2174/1389450054021918. Curr Drug Targets. 2005. PMID: 16026258 Review.
Cited by
-
Fibroblast growth factor 9 (FGF9) inhibits myogenic differentiation of C2C12 and human muscle cells.Cell Cycle. 2019 Dec;18(24):3562-3580. doi: 10.1080/15384101.2019.1691796. Epub 2019 Nov 18. Cell Cycle. 2019. PMID: 31735119 Free PMC article.
-
Tuning Myogenesis by Controlling Gelatin Hydrogel Properties through Hydrogen Peroxide-Mediated Cross-Linking and Degradation.Gels. 2022 Jun 17;8(6):387. doi: 10.3390/gels8060387. Gels. 2022. PMID: 35735731 Free PMC article.
-
Myogenic Cell Expression of Intercellular Adhesion Molecule-1 Contributes to Muscle Regeneration after Injury.Am J Pathol. 2020 Oct;190(10):2039-2055. doi: 10.1016/j.ajpath.2020.06.009. Epub 2020 Jul 8. Am J Pathol. 2020. PMID: 32650005 Free PMC article.
-
Hypoxia-induced PD-L1 expression and modulation of muscle stem cell allograft rejection.Front Pharmacol. 2024 Nov 1;15:1471563. doi: 10.3389/fphar.2024.1471563. eCollection 2024. Front Pharmacol. 2024. PMID: 39555101 Free PMC article.
-
Regenerating Myofibers after an Acute Muscle Injury: What Do We Really Know about Them?Int J Mol Sci. 2023 Aug 8;24(16):12545. doi: 10.3390/ijms241612545. Int J Mol Sci. 2023. PMID: 37628725 Free PMC article. Review.
References
-
- Pavlath GK. Current Progress Towards Understanding Mechanisms of Myoblast Fusion in Mammals. Springer-Verlag; Berlin, Heidelberger Platz 3, D–14197 Berlin, Germany: 2011.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

