A phase II study of laquinimod in Crohn's disease
- PMID: 25281416
- PMCID: PMC4515993
- DOI: 10.1136/gutjnl-2014-307118
A phase II study of laquinimod in Crohn's disease
Abstract
Objective: Laquinimod is an oral therapeutic agent under investigation for the treatment of Crohn's disease (CD), Huntington's disease, lupus nephritis and multiple sclerosis. This dose escalation study evaluated the safety and efficacy of laquinimod as induction therapy in patients with active moderate-severe CD.
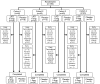
Design: Multicentre, double-blind, sequential-cohort, randomised controlled trial with laquinimod doses of 0.5, 1, 1.5 or 2 mg/day or placebo (n=45 per cohort randomised in a 2:1 ratio) for 8 weeks with 4-week follow-up. Stable concomittant therapies and prior use of anti-tumour necrosis factor agents were permitted. Comprehensive safety assessments were performed and efficacy analyses included the proportions of patients in clinical remission (CD Activity Index (CDAI) <150 and no treatment failure (TF)), and with a clinical response (70 or 100 point CDAI reduction from baseline or remission and no TF).
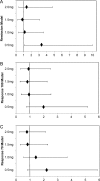
Results: 117 patients received laquinimod and 63 patients received placebo. The overall incidence of adverse events (AEs) in the laquinimod group was similar to the pooled placebo group (86.2%-96.7% vs 82.5%) and most AEs were mild to moderate in severity. Treatment with laquinimod 0.5 mg showed consistent effects on remission (48.3% (CI 31% to 66%) vs 15.9% (CI 9% to 27%)), response 100 (55.2% (CI 37% to 71%) vs 31.7% (CI 22% to 44%)) and response 70 (62.1% (CI 44% to 77%) vs 34.9% (CI 24% to 47%)) versus placebo. Laquinimod 1.0 mg showed less benefit (26.7% remission (CI 14% to 44%) and 53.3% response 70 (CI 36% to 70%)), and no effect was noted on remission/response at higher doses.
Conclusions: Laquinimod was safe and well tolerated, and the effects on remission and response of the 0.5 mg dose suggest a treatment benefit in patients with CD.
Trial registration number: NCT00737932.
Keywords: CLINICAL DECISION MAKING; CLINICAL TRIALS; CROHN'S DISEASE; IBD CLINICAL; PHARMACOTHERAPY.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures

References
-
- Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn's disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol 2007;5:1424–9. - PubMed
-
- Cohen RD. The quality of life in patients with Crohn's disease. Aliment Pharmacol Ther 2002;16:1603–9. - PubMed
-
- Odes S, Vardi H, Friger M, et al. Cost analysis and cost determinants in a European inflammatory bowel disease inception cohort with 10 years of follow-up evaluation. Gastroenterology 2006;131:719–28. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
