Focused chemical genomics using zebrafish xenotransplantation as a pre-clinical therapeutic platform for T-cell acute lymphoblastic leukemia
- PMID: 25281505
- PMCID: PMC4281315
- DOI: 10.3324/haematol.2014.110742
Focused chemical genomics using zebrafish xenotransplantation as a pre-clinical therapeutic platform for T-cell acute lymphoblastic leukemia
Abstract
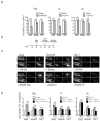
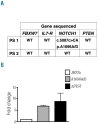
Cancer therapeutics is evolving to precision medicine, with the goal of matching targeted compounds with molecular aberrations underlying a patient's cancer. While murine models offer a pre-clinical tool, associated costs and time are not compatible with actionable patient-directed interventions. Using the paradigm of T-cell acute lymphoblastic leukemia, a high-risk disease with defined molecular underpinnings, we developed a zebrafish human cancer xenotransplantation model to inform therapeutic decisions. Using a focused chemical genomic approach, we demonstrate that xenografted cell lines harboring mutations in the NOTCH1 and PI3K/AKT pathways respond concordantly to their targeted therapies, patient-derived T-cell acute lymphoblastic leukemia can be successfully engrafted in zebrafish and specific drug responses can be quantitatively determined. Using this approach, we identified a mutation sensitive to γ-secretase inhibition in a xenograft from a child with T-cell acute lymphoblastic leukemia, confirmed by Sanger sequencing and validated as a gain-of-function NOTCH1 mutation. The zebrafish xenotransplantation platform provides a novel cost-effective means of tailoring leukemia therapy in real time.
Copyright© Ferrata Storti Foundation.
Figures

Similar articles
-
The relevance of PTEN-AKT in relation to NOTCH1-directed treatment strategies in T-cell acute lymphoblastic leukemia.Haematologica. 2016 Sep;101(9):1010-7. doi: 10.3324/haematol.2016.146381. Haematologica. 2016. PMID: 27582570 Free PMC article. Review.
-
The significance of PTEN and AKT aberrations in pediatric T-cell acute lymphoblastic leukemia.Haematologica. 2012 Sep;97(9):1405-13. doi: 10.3324/haematol.2011.059030. Epub 2012 Apr 4. Haematologica. 2012. PMID: 22491738 Free PMC article.
-
Regulation of PTEN by CK2 and Notch1 in primary T-cell acute lymphoblastic leukemia: rationale for combined use of CK2- and gamma-secretase inhibitors.Haematologica. 2010 Apr;95(4):674-8. doi: 10.3324/haematol.2009.011999. Epub 2009 Dec 16. Haematologica. 2010. PMID: 20015880 Free PMC article.
-
New insights into Notch1 regulation of the PI3K-AKT-mTOR1 signaling axis: targeted therapy of γ-secretase inhibitor resistant T-cell acute lymphoblastic leukemia.Cell Signal. 2014 Jan;26(1):149-61. doi: 10.1016/j.cellsig.2013.09.021. Epub 2013 Oct 16. Cell Signal. 2014. PMID: 24140475 Review.
-
The Notch driven long non-coding RNA repertoire in T-cell acute lymphoblastic leukemia.Haematologica. 2014 Dec;99(12):1808-16. doi: 10.3324/haematol.2014.115683. Epub 2014 Oct 24. Haematologica. 2014. PMID: 25344525 Free PMC article.
Cited by
-
Zebrafish xenograft as a tool for the study of colorectal cancer: a review.Cell Death Dis. 2024 Jan 9;15(1):23. doi: 10.1038/s41419-023-06291-0. Cell Death Dis. 2024. PMID: 38195619 Free PMC article. Review.
-
Modeling Cancer Using Zebrafish Xenografts: Drawbacks for Mimicking the Human Microenvironment.Cells. 2020 Aug 27;9(9):1978. doi: 10.3390/cells9091978. Cells. 2020. PMID: 32867288 Free PMC article. Review.
-
Investigating Cutaneous Squamous Cell Carcinoma in vitro and in vivo: Novel 3D Tools and Animal Models.Front Med (Lausanne). 2022 May 9;9:875517. doi: 10.3389/fmed.2022.875517. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35646967 Free PMC article. Review.
-
Zebrafish Avatars towards Personalized Medicine-A Comparative Review between Avatar Models.Cells. 2020 Jan 25;9(2):293. doi: 10.3390/cells9020293. Cells. 2020. PMID: 31991800 Free PMC article. Review.
-
Zebrafish phenotypic screen identifies novel Notch antagonists.Invest New Drugs. 2017 Apr;35(2):166-179. doi: 10.1007/s10637-016-0423-y. Epub 2017 Jan 5. Invest New Drugs. 2017. PMID: 28058624
References
-
- Weng AP, Ferrando AA, Lee W, Morris JP, Silverman LB, Sanchez-Irizarry C, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306(5694):269–71. - PubMed
-
- Vrooman LM, Silverman LB. Childhood acute lymphoblastic leukemia: update on prognostic factors. Curr Opin Pediatr. 2009;21(1):1–8. - PubMed
-
- Grabher C, von Boehmer H, Look a T. Notch 1 activation in the molecular pathogenesis of T-cell acute lymphoblastic leukaemia. Nat Rev Cancer. 2006;6(5):347–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

