Industry sponsorship bias in research findings: a network meta-analysis of LDL cholesterol reduction in randomised trials of statins
- PMID: 25281681
- PMCID: PMC4184241
- DOI: 10.1136/bmj.g5741
Industry sponsorship bias in research findings: a network meta-analysis of LDL cholesterol reduction in randomised trials of statins
Abstract
Objective: To explore the risk of industry sponsorship bias in a systematically identified set of placebo controlled and active comparator trials of statins.
Design: Systematic review and network meta-analysis.
Eligibility: Open label and double blind randomised controlled trials comparing one statin with another at any dose or with control (placebo, diet, or usual care) for adults with, or at risk of developing, cardiovascular disease. Only trials that lasted longer than four weeks with more than 50 participants per trial arm were included. Two investigators assessed study eligibility.
Data sources: Bibliographic databases and reference lists of relevant articles published between 1 January 1985 and 10 March 2013.
Data extraction: One investigator extracted data and another confirmed accuracy.
Main outcome measure: Mean absolute change from baseline concentration of low density lipoprotein (LDL) cholesterol.
Data synthesis: Study level outcomes from randomised trials were combined using random effects network meta-analyses.
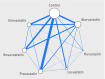
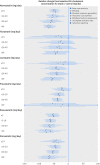
Results: We included 183 randomised controlled trials of statins, 103 of which were two-armed or multi-armed active comparator trials. When all of the existing randomised evidence was synthesised in network meta-analyses, there were clear differences in the LDL cholesterol lowering effects of individual statins at different doses. In general, higher doses resulted in higher reductions in baseline LDL cholesterol levels. Of a total of 146 industry sponsored trials, 64 were placebo controlled (43.8%). The corresponding number for the non-industry sponsored trials was 16 (43.2%). Of the 35 unique comparisons available in 37 non-industry sponsored trials, 31 were also available in industry sponsored trials. There were no systematic differences in magnitude between the LDL cholesterol lowering effects of individual statins observed in industry sponsored versus non-industry sponsored trials. In industry sponsored trials, the mean change from baseline LDL cholesterol level was on average 1.77 mg/dL (95% credible interval -11.12 to 7.66) lower than the change observed in non-industry sponsored trials. There was no detectable inconsistency in the evidence network.
Conclusions: Our analysis shows that the findings obtained from industry sponsored statin trials seem similar in magnitude as those in non-industry sources. There are actual differences in the effectiveness of individual statins at various doses that explain previously observed discrepancies between industry and non-industry sponsored trials.
© Naci et al 2014.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures




Comment in
-
Bias related to funding source in statin trials.BMJ. 2014 Oct 3;349:g5949. doi: 10.1136/bmj.g5949. BMJ. 2014. PMID: 25281682 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
