Differentially phased leaf growth and movements in Arabidopsis depend on coordinated circadian and light regulation
- PMID: 25281688
- PMCID: PMC4247567
- DOI: 10.1105/tpc.114.129031
Differentially phased leaf growth and movements in Arabidopsis depend on coordinated circadian and light regulation
Abstract
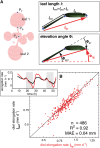
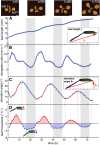
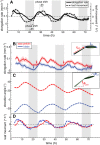
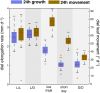
In contrast to vastly studied hypocotyl growth, little is known about diel regulation of leaf growth and its coordination with movements such as changes in leaf elevation angle (hyponasty). We developed a 3D live-leaf growth analysis system enabling simultaneous monitoring of growth and movements. Leaf growth is maximal several hours after dawn, requires light, and is regulated by daylength, suggesting coupling between growth and metabolism. We identify both blade and petiole positioning as important components of leaf movements in Arabidopsis thaliana and reveal a temporal delay between growth and movements. In hypocotyls, the combination of circadian expression of PHYTOCHROME INTERACTING FACTOR4 (PIF4) and PIF5 and their light-regulated protein stability drives rhythmic hypocotyl elongation with peak growth at dawn. We find that PIF4 and PIF5 are not essential to sustain rhythmic leaf growth but influence their amplitude. Furthermore, EARLY FLOWERING3, a member of the evening complex (EC), is required to maintain the correct phase between growth and movement. Our study shows that the mechanisms underlying rhythmic hypocotyl and leaf growth differ. Moreover, we reveal the temporal relationship between leaf elongation and movements and demonstrate the importance of the EC for the coordination of these phenotypic traits.
© 2014 American Society of Plant Biologists. All rights reserved.
Figures

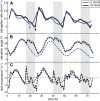
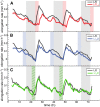
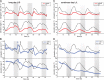
Comment in
-
Simultaneous monitoring of leaf growth and leaf movement.Plant Cell. 2014 Oct;26(10):3828. doi: 10.1105/tpc.114.133728. Epub 2014 Oct 31. Plant Cell. 2014. PMID: 25361957 Free PMC article. No abstract available.
References
-
- Andriankaja M., Dhondt S., De Bodt S., Vanhaeren H., Coppens F., De Milde L., Mühlenbock P., Skirycz A., Gonzalez N., Beemster G.T., Inzé D. (2012). Exit from proliferation during leaf development in Arabidopsis thaliana: a not-so-gradual process. Dev. Cell 22: 64–78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

