Fibrocytes are not an essential source of type I collagen during lung fibrosis
- PMID: 25281715
- PMCID: PMC4233459
- DOI: 10.4049/jimmunol.1400753
Fibrocytes are not an essential source of type I collagen during lung fibrosis
Abstract
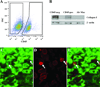
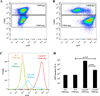
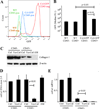
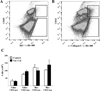
Progressive fibrosis involves accumulation of activated collagen-producing mesenchymal cells. Fibrocytes are hematopoietic-derived cells with mesenchymal features that potentially have a unique and critical function during fibrosis. Fibrocytes have been proposed as an important direct contributor of type I collagen deposition during fibrosis based largely on fate-mapping studies. To determine the functional contribution of hematopoietic cell-derived type I collagen to fibrogenesis, we use a double-transgenic system to specifically delete the type I collagen gene across a broad population of hematopoietic cells. These mice develop a robust fibrotic response similar to littermate genotype control mice injured with bleomycin indicating that fibrocytes are not a necessary source of type I collagen. Using collagen-promoter GFP mice, we find that fibrocytes express type I collagen. However, fibrocytes with confirmed deletion of the type I collagen gene have readily detectable intracellular type I collagen indicating that uptake of collagen from neighboring cells account for much of the fibrocyte collagen. Collectively, these results clarify several seemingly conflicting reports regarding the direct contribution of fibrocytes to collagen deposition.
Copyright © 2014 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors have no conflicting financial interests.
Figures




References
-
- American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–664. - PubMed
-
- American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. - PubMed
-
- Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. - PubMed
-
- Thannickal VJ, Toews GB, White ES, Lynch JP, 3rd, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

