Monoclonal antibodies against Aβ42 fibrils distinguish multiple aggregation state polymorphisms in vitro and in Alzheimer disease brain
- PMID: 25281743
- PMCID: PMC4231689
- DOI: 10.1074/jbc.M114.594846
Monoclonal antibodies against Aβ42 fibrils distinguish multiple aggregation state polymorphisms in vitro and in Alzheimer disease brain
Abstract
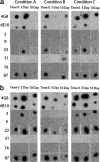
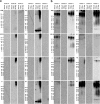
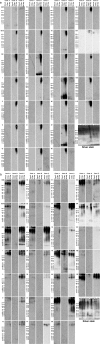
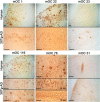
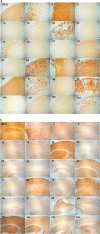
Amyloidogenic proteins generally form intermolecularly hydrogen-bonded β-sheet aggregates, including parallel, in-register β-sheets (recognized by antiserum OC) or antiparallel β-sheets, β-solenoids, β-barrels, and β-cylindrins (recognized by antiserum A11). Although these groups share many common properties, some amyloid sequences have been reported to form polymorphic structural variants or strains. We investigated the humoral immune response to Aβ42 fibrils and produced 23 OC-type monoclonal antibodies recognizing distinct epitopes differentially associated with polymorphic structural variants. These mOC antibodies define at least 18 different immunological profiles represented in aggregates of amyloid-β (Aβ). All of the antibodies strongly prefer amyloid aggregates over monomer, indicating that they recognize conformational epitopes. Most of the antibodies react with N-terminal linear segments of Aβ, although many recognize a discontinuous epitope consisting of an N-terminal domain and a central domain. Several of the antibodies that recognize linear Aβ segments also react with fibrils formed from unrelated amyloid sequences, indicating that reactivity with linear segments of Aβ does not mean the antibody is sequence-specific. The antibodies display strikingly different patterns of immunoreactivity in Alzheimer disease and transgenic mouse brain and identify spatially and temporally unique amyloid deposits. Our results indicate that the immune response to Aβ42 fibrils is diverse and reflects the structural polymorphisms in fibrillar amyloid structures. These polymorphisms may contribute to differences in toxicity and consequent effects on pathological processes. Thus, a single therapeutic monoclonal antibody may not be able to target all of the pathological aggregates necessary to make an impact on the overall disease process.
Keywords: Alzheimer Disease; Amyloid; Aβ; Monoclonal Antibody; Peptide Conformation; Protein Aggregation.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Querfurth H. W., LaFerla F. M. (2010) Alzheimer's disease. N. Engl. J. Med. 362, 329–344 - PubMed
-
- Hebert L. E., Scherr P. A., Bienias J. L., Bennett D. A., Evans D. A. (2003) Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch. Neurol. 60, 1119–1122 - PubMed
-
- Katzman R., Saitoh T. (1991) Advances in Alzheimer's disease. FASEB J. 5, 278–286 - PubMed
-
- Hardy J., Selkoe D. J. (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297, 353–356 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

