The programmed death-1 immune-suppressive pathway: barrier to antitumor immunity
- PMID: 25281753
- PMCID: PMC4185425
- DOI: 10.4049/jimmunol.1401572
The programmed death-1 immune-suppressive pathway: barrier to antitumor immunity
Abstract
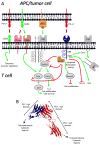
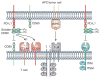
Programmed death ligand 1 (PD-L1, also known as B7 homolog 1 or CD274) is a major obstacle to antitumor immunity because it tolerizes/anergizes tumor-reactive T cells by binding to its receptor programmed death-1 (CD279), renders tumor cells resistant to CD8(+) T cell- and FasL-mediated lysis, and tolerizes T cells by reverse signaling through T cell-expressed CD80. PD-L1 is abundant in the tumor microenvironment, where it is expressed by many malignant cells, as well as by immune cells and vascular endothelial cells. The critical role of PD-L1 in obstructing antitumor immunity has been demonstrated in multiple animal models and in recent clinical trials. This article reviews the mechanisms by which PD-L1 impairs antitumor immunity and discusses established and experimental strategies for maintaining T cell activation in the presence of PD-L1-expressing cells in the tumor microenvironment.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures

References
-
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. - PMC - PubMed
-
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. - PMC - PubMed
-
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. - PMC - PubMed
-
- Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, Leming PD, Lipson EJ, Puzanov I, Smith DC, Taube JM, Wigginton JM, Kollia GD, Gupta A, Pardoll DM, Sosman JA, Hodi FS. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–1030. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

