Expanding the results of a high throughput screen against an isochorismate-pyruvate lyase to enzymes of a similar scaffold or mechanism
- PMID: 25282647
- PMCID: PMC4254016
- DOI: 10.1016/j.bmc.2014.09.010
Expanding the results of a high throughput screen against an isochorismate-pyruvate lyase to enzymes of a similar scaffold or mechanism
Abstract
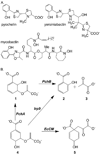
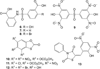
Antibiotic resistance is a growing health concern, and new avenues of antimicrobial drug design are being actively sought. One suggested pathway to be targeted for inhibitor design is that of iron scavenging through siderophores. Here we present a high throughput screen to the isochorismate-pyruvate lyase of Pseudomonas aeruginosa, an enzyme required for the production of the siderophore pyochelin. Compounds identified in the screen are high nanomolar to low micromolar inhibitors of the enzyme and produce growth inhibition in PAO1 P. aeruginosa in the millimolar range under iron-limiting conditions. The identified compounds were also tested for enzymatic inhibition of Escherichia coli chorismate mutase, a protein of similar fold and similar chemistry, and of Yersinia enterocolitica salicylate synthase, a protein of differing fold but catalyzing the same lyase reaction. In both cases, subsets of the inhibitors from the screen were found to be inhibitory to enzymatic activity (mutase or synthase) in the micromolar range and capable of growth inhibition in their respective organisms (E. coli or Y. enterocolitica).
Keywords: Chorismate mutase; Isochorismate-pyruvate lyase; Salicylate synthase; Siderophore.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Investigation of potential inhibitors of chorismate-utilizing enzymes.Curr Med Chem. 2015;22(11):1383-99. doi: 10.2174/0929867322666150209152446. Curr Med Chem. 2015. PMID: 25666801 Review.
-
Irp9, encoded by the high-pathogenicity island of Yersinia enterocolitica, is able to convert chorismate into salicylate, the precursor of the siderophore yersiniabactin.J Bacteriol. 2003 Sep;185(18):5648-53. doi: 10.1128/JB.185.18.5648-5653.2003. J Bacteriol. 2003. PMID: 12949119 Free PMC article.
-
Exploration of swapping enzymatic function between two proteins: a simulation study of chorismate mutase and isochorismate pyruvate lyase.Protein Sci. 2013 Jun;22(6):809-22. doi: 10.1002/pro.2264. Protein Sci. 2013. PMID: 23595942 Free PMC article.
-
Lysine221 is the general base residue of the isochorismate synthase from Pseudomonas aeruginosa (PchA) in a reaction that is diffusion limited.Arch Biochem Biophys. 2013 Oct 1;538(1):49-56. doi: 10.1016/j.abb.2013.07.026. Epub 2013 Aug 11. Arch Biochem Biophys. 2013. PMID: 23942051 Free PMC article.
-
Mechanistic and inhibition studies of chorismate-utilizing enzymes.Biochem Soc Trans. 2005 Aug;33(Pt 4):763-6. doi: 10.1042/BST0330763. Biochem Soc Trans. 2005. PMID: 16042594 Review.
Cited by
-
Breaking a pathogen's iron will: Inhibiting siderophore production as an antimicrobial strategy.Biochim Biophys Acta. 2015 Aug;1854(8):1054-70. doi: 10.1016/j.bbapap.2015.05.001. Epub 2015 May 10. Biochim Biophys Acta. 2015. PMID: 25970810 Free PMC article. Review.
-
Kinetic analysis of the three-substrate reaction mechanism of an NRPS-independent siderophore (NIS) synthetase.Methods Enzymol. 2024;702:1-19. doi: 10.1016/bs.mie.2024.06.012. Epub 2024 Jul 3. Methods Enzymol. 2024. PMID: 39155107 Free PMC article.
-
Unraveling the Structure and Mechanism of the MST(ery) Enzymes.Trends Biochem Sci. 2018 May;43(5):342-357. doi: 10.1016/j.tibs.2018.02.011. Epub 2018 Mar 21. Trends Biochem Sci. 2018. PMID: 29573882 Free PMC article. Review.
-
Iron Acquisition and Metabolism as a Promising Target for Antimicrobials (Bottlenecks and Opportunities): Where Do We Stand?Int J Mol Sci. 2023 Mar 24;24(7):6181. doi: 10.3390/ijms24076181. Int J Mol Sci. 2023. PMID: 37047161 Free PMC article. Review.
-
An Open and Shut Case: The Interaction of Magnesium with MST Enzymes.J Am Chem Soc. 2016 Jul 27;138(29):9277-93. doi: 10.1021/jacs.6b05134. Epub 2016 Jul 19. J Am Chem Soc. 2016. PMID: 27373320 Free PMC article.
References
-
- Bearden SW, Perry RD. The Yfe system of Yersinia pestis transports iron and manganese and is required for full virulence of plague. Mol Microbiol. 1999;32(2):403–414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical
Molecular Biology Databases

