Validation of association of the apolipoprotein E ε2 allele with neurodevelopmental dysfunction after cardiac surgery in neonates and infants
- PMID: 25282659
- PMCID: PMC4376113
- DOI: 10.1016/j.jtcvs.2014.07.052
Validation of association of the apolipoprotein E ε2 allele with neurodevelopmental dysfunction after cardiac surgery in neonates and infants
Abstract
Objective: Apolipoprotein E (APOE) genotype is a determinant of neurologic recovery after brain ischemia and traumatic brain injury. The APOE ε2 allele has been associated with worse neurodevelopmental (ND) outcome after repair of congenital heart defects (CHD) in infancy. Replication of this finding in an independent cohort is essential to validate the observed genotype-phenotype association.
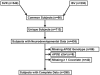
Methods: The association of APOE genotype with ND outcomes was assessed in a combined cohort of patients with single-ventricle CHD enrolled in the Single Ventricle Reconstruction and Infant Single Ventricle trials. ND outcome was assessed at 14 months using the Psychomotor Development Index (PDI) and Mental Development Index (MDI) of the Bayley Scales of Infant Development-II. Stepwise multivariable regression was performed to develop predictive models for PDI and MDI scores.
Results: Complete data were available for 298 of 435 patients. After adjustment for preoperative and postoperative covariates, the APOE ε2 allele was associated with a lower PDI score (P = .038). Patients with the ε2 allele had a PDI score approximately 6 points lower than those without the risk allele, explaining 1.04% of overall PDI variance, because the ε2 allele was present in only 11% of the patients. There was a marginal effect of the ε2 allele on MDI scores (P = .058).
Conclusions: These data validate the association of the APOE ε2 allele with adverse early ND outcomes after cardiac surgery in infants, independent of patient and operative factors. Genetic variants that decrease neuroresilience and impair neuronal repair after brain injury are important risk factors for ND dysfunction after surgery for CHD.
Copyright © 2014. Published by Elsevier Inc.
Figures
Comment in
-
Discussion.J Thorac Cardiovasc Surg. 2014 Dec;148(6):2566-8. doi: 10.1016/j.jtcvs.2014.07.055. Epub 2014 Oct 1. J Thorac Cardiovasc Surg. 2014. PMID: 25282658 No abstract available.
-
Validation accepted, but look at what else was revealed.J Thorac Cardiovasc Surg. 2014 Dec;148(6):2568-9. doi: 10.1016/j.jtcvs.2014.10.008. Epub 2014 Oct 7. J Thorac Cardiovasc Surg. 2014. PMID: 25433876 No abstract available.
References
-
- Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management. A scientific statement from the American Heart Association. Circulation. 2012;126:1143–72. - PubMed
-
- Ballweg JA, Wernovsky G, Gaynor JW. Neurodevelopmental outcomes following congenital heart surgery. Pediatr Cardiol. 2006;28:1450–9. - PubMed
-
- Shillingford AJ, Glanzman MM, Ittenbach RF, Clancy RR, Gaynor JW, Wernovsky G. Inattention, hyperactivity, and school performance in a population of school-age children with complex congenital heart disease. Pediatrics. 2008;121:e759–67. - PubMed
-
- Tabbutt S, Gaynor JW, Newburger JW. Neurodevelopmental outcomes after congenital heart surgery and strategies for improvement. Curr Opin Cardiol. 2012;27:82–91. - PubMed
-
- Fuller S, Nord AS, Gerdes M, Wernovsky G, Jarvik GP, Bernbaum J, et al. Pre-dictors of impaired neurodevelopmental outcomes at one year of age after infant cardiac surgery. Eur J Cardiothorac Surg. 2009;36:40–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL068288/HL/NHLBI NIH HHS/United States
- HL068285/HL/NHLBI NIH HHS/United States
- U10 HL109743/HL/NHLBI NIH HHS/United States
- U01 HL068290/HL/NHLBI NIH HHS/United States
- U10 HL068270/HL/NHLBI NIH HHS/United States
- U01 HL068281/HL/NHLBI NIH HHS/United States
- UL1 RR025758/RR/NCRR NIH HHS/United States
- UL1 RR 025758/RR/NCRR NIH HHS/United States
- U01 HL068269/HL/NHLBI NIH HHS/United States
- F31 MH101905/MH/NIMH NIH HHS/United States
- U10 HL109816/HL/NHLBI NIH HHS/United States
- P30 HD018655/HD/NICHD NIH HHS/United States
- U01 HL068279/HL/NHLBI NIH HHS/United States
- HL068279/HL/NHLBI NIH HHS/United States
- U01 HL068288/HL/NHLBI NIH HHS/United States
- U01 HL068270/HL/NHLBI NIH HHS/United States
- HL085057/HL/NHLBI NIH HHS/United States
- HL068281/HL/NHLBI NIH HHS/United States
- U01 HL068292/HL/NHLBI NIH HHS/United States
- HL068269/HL/NHLBI NIH HHS/United States
- HL068270/HL/NHLBI NIH HHS/United States
- 1F31MH101905-01/MH/NIMH NIH HHS/United States
- U01 HL085057/HL/NHLBI NIH HHS/United States
- U10 HL109778/HL/NHLBI NIH HHS/United States
- HL068290/HL/NHLBI NIH HHS/United States
- U10 HL109673/HL/NHLBI NIH HHS/United States
- U01 HL068285/HL/NHLBI NIH HHS/United States
- HL068292/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous