Amyloid precursor protein (APP) affects global protein synthesis in dividing human cells
- PMID: 25283437
- PMCID: PMC4445069
- DOI: 10.1002/jcp.24835
Amyloid precursor protein (APP) affects global protein synthesis in dividing human cells
Abstract
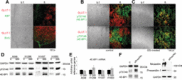
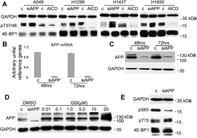
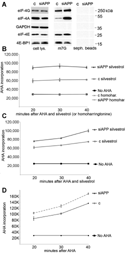
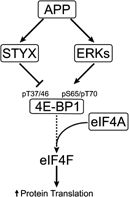
Hypoxic non-small cell lung cancer (NSCLC) is dependent on Notch-1 signaling for survival. Targeting Notch-1 by means of γ-secretase inhibitors (GSI) proved effective in killing hypoxic NSCLC. Post-mortem analysis of GSI-treated, NSCLC-burdened mice suggested enhanced phosphorylation of 4E-BP1 at threonines 37/46 in hypoxic tumor tissues. In vitro dissection of this phenomenon revealed that Amyloid Precursor Protein (APP) inhibition was responsible for a non-canonical 4E-BP1 phosphorylation pattern rearrangement-a process, in part, mediated by APP regulation of the pseudophosphatase Styx. Upon APP depletion we observed modifications of eIF-4F composition indicating increased recruitment of eIF-4A to the mRNA cap. This phenomenon was supported by the observation that cells with depleted APP were partially resistant to silvestrol, an antibiotic that interferes with eIF-4A assembly into eIF-4F complexes. APP downregulation in dividing human cells increased the rate of global protein synthesis, both cap- and IRES-dependent. Such an increase seemed independent of mTOR inhibition. After administration of Torin-1, APP downregulation and Mechanistic Target of Rapamycin Complex 1 (mTORC-1) inhibition affected 4E-BP1 phosphorylation and global protein synthesis in opposite fashions. Additional investigations indicated that APP operates independently of mTORC-1. Key phenomena described in this study were reversed by overexpression of the APP C-terminal domain. The presented data suggest that APP may be a novel regulator of protein synthesis in dividing human cells, both cancerous and primary. Furthermore, APP appears to affect translation initiation using mechanisms seemingly dissimilar to mTORC-1 regulation of cap-dependent protein synthesis.
© 2014 Wiley Periodicals, Inc.
Figures


References
-
- Bachmair A, Finley D, Varshavsky A. 1986. In vivo half‐life of a protein is a function of its amino‐terminal residue. Science 234:179–186. - PubMed
-
- Beckett C, Nalivaeva NN, Belyaev ND, Turner AJ. 2012. Nuclear signalling by membrane protein intracellular domains: The AICD enigma. Cell Signal 24:402–409. - PubMed
-
- Böhni R, Riesgo‐Escovar J, Oldham S, Brogiolo W, Stocker H, Andruss BF, Beckingham K, Hafen E. 1996. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1–4. Cell 97:865–875. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

