Coreceptor scanning by the T cell receptor provides a mechanism for T cell tolerance
- PMID: 25284152
- PMCID: PMC4304671
- DOI: 10.1016/j.cell.2014.08.042
Coreceptor scanning by the T cell receptor provides a mechanism for T cell tolerance
Abstract
In the thymus, high-affinity, self-reactive thymocytes are eliminated from the pool of developing T cells, generating central tolerance. Here, we investigate how developing T cells measure self-antigen affinity. We show that very few CD4 or CD8 coreceptor molecules are coupled with the signal-initiating kinase, Lck. To initiate signaling, an antigen-engaged T cell receptor (TCR) scans multiple coreceptor molecules to find one that is coupled to Lck; this is the first and rate-limiting step in a kinetic proofreading chain of events that eventually leads to TCR triggering and negative selection. MHCII-restricted TCRs require a shorter antigen dwell time (0.2 s) to initiate negative selection compared to MHCI-restricted TCRs (0.9 s) because more CD4 coreceptors are Lck-loaded compared to CD8. We generated a model (Lck come&stay/signal duration) that accurately predicts the observed differences in antigen dwell-time thresholds used by MHCI- and MHCII-restricted thymocytes to initiate negative selection and generate self-tolerance.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures



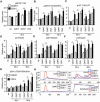
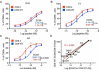
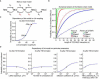
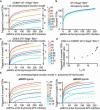
Comment in
-
Immunology: Tolerance lies in the timing.Nature. 2014 Nov 27;515(7528):502-3. doi: 10.1038/515502a. Nature. 2014. PMID: 25428497 No abstract available.
References
-
- Daniels MA, Teixeiro E, Gill J, Hausmann B, Roubaty D, Holmberg K, Werlen G, Hollander GA, Gascoigne NR, Palmer E. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. - PubMed
-
- Dustin ML, Bromley SK, Davis MM, Zhu C. Identification of self through two-dimensional chemistry and synapses. Annu Rev Cell Dev Biol. 2001;17:133–157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

