Assembly dynamics and the roles of FliI ATPase of the bacterial flagellar export apparatus
- PMID: 25284201
- PMCID: PMC4185386
- DOI: 10.1038/srep06528
Assembly dynamics and the roles of FliI ATPase of the bacterial flagellar export apparatus
Abstract
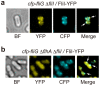
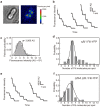
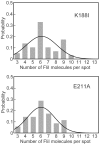
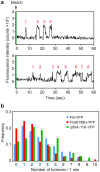
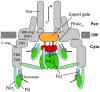
For construction of the bacterial flagellum, FliI ATPase forms the FliH2-FliI complex in the cytoplasm and localizes to the flagellar basal body (FBB) through the interaction of FliH with a C ring protein, FliN. FliI also assembles into a homo-hexamer to promote initial entry of export substrates into the export gate. The interaction of FliH with an export gate protein, FlhA, is required for stable anchoring of the FliI6 ring to the gate. Here we report the stoichiometry and assembly dynamics of FliI-YFP by fluorescence microscopy with single molecule precision. More than six FliI-YFP molecules were associated with the FBB through interactions of FliH with FliN and FlhA. Single FliI-YFP molecule exchanges between the FBB-localized and free-diffusing ones were observed several times per minute. Neither the number of FliI-YFP associated with the FBB nor FliI-YFP turnover rate were affected by catalytic mutations in FliI, indicating that ATP hydrolysis by FliI does not drive the assembly-disassembly cycle of FliI during flagellar assembly. We propose that the FliH2FliI complex and FliI6 ring function as a dynamic substrate carrier and a static substrate loader, respectively.
Figures
References
-
- Minamino T., Imada K. & Namba K. Mechanisms of type III protein export for bacterial flagellar assembly. Mol. BioSyst. 4, 1105–1115 (2008). - PubMed
-
- Minamino T. Protein export through the bacterial flagellar type III export pathway. Biochim. Biophys. Acta. 1843, 1642–1648 (2014). - PubMed
-
- Francis N. R., Sosinsky G. E., Thomas D. & DeRosier D. J. Isolation, characterization, and structure of bacterial flagellar motors containing the switch complex. J. Mol. Biol. 235, 1261–1270 (1994). - PubMed
-
- González-Pedrajo B., Minamino T., Kihara M. & Namba K. Interactions between C ring proteins and export apparatus components: a possible mechanism for facilitating type III protein export. Mol. Microbiol. 60, 984–998 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

