Metabolic reprogramming during TGFβ1-induced epithelial-to-mesenchymal transition
- PMID: 25284588
- PMCID: PMC4387121
- DOI: 10.1038/onc.2014.321
Metabolic reprogramming during TGFβ1-induced epithelial-to-mesenchymal transition
Abstract
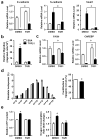
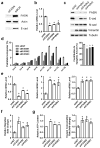
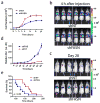
Metastatic progression, including extravasation and micrometastatic outgrowth, is the main cause of cancer patient death. Recent studies suggest that cancer cells reprogram their metabolism to support increased proliferation through increased glycolysis and biosynthetic activities, including lipogenesis pathways. However, metabolic changes during metastatic progression, including alterations in regulatory gene expression, remain undefined. We show that transforming growth factor beta 1 (TGFβ1)-induced epithelial-to-mesenchymal transition (EMT) is accompanied by coordinately reduced enzyme expression required to convert glucose into fatty acids, and concomitant enhanced respiration. Overexpressed Snail1, a transcription factor mediating TGFβ1-induced EMT, was sufficient to suppress carbohydrate-responsive-element-binding protein (ChREBP, a master lipogenic regulator), and fatty acid synthase (FASN), its effector lipogenic gene. Stable FASN knockdown was sufficient to induce EMT, stimulate migration and extravasation in vitro. FASN silencing enhanced lung metastasis and death in vivo. These data suggest that a metabolic transition that suppresses lipogenesis and favors energy production is an essential component of TGFβ1-induced EMT and metastasis.
Figures


References
-
- Pallis AG, Syrigos K. Targeted (and chemotherapeutic) agents as maintenance treatment in patients with metastatic non-small-cell lung cancer: current status and future challenges. Cancer treatment reviews. 2012 Nov;38(7):861–7. - PubMed
-
- Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005 Aug 29;24(37):5764–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

