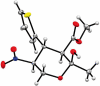
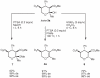
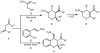Asymmetric Synthesis of Functionalized Dihydro- and Tetrahydropyrans via an Organocatalytic Domino Michael-Hemiacetalization Reaction
- PMID: 25284900
- PMCID: PMC4181541
- DOI: 10.1055/s-0033-1340826
Asymmetric Synthesis of Functionalized Dihydro- and Tetrahydropyrans via an Organocatalytic Domino Michael-Hemiacetalization Reaction
Abstract
Starting from α-hydroxymethyl nitroalkenes and various 1,3-dicarbonyl compounds, a one-pot organocatalyzed diastereo- and enantioselective synthesis of polyfunctionalized dihydro- and tetrahydropyran derivatives via a domino Michael-hemiacetalization sequence is reported. The title compounds bearing a variety of functional groups can be synthesized in this way in good yields (59-91%) and with moderate to excellent diastereoselectivities (26-98% de) and enantioselectivities (71-99% ee).
Keywords: domino reaction; hydrogen bonding; one-pot reaction; organocatalysis; tetrahydropyrans.
Figures
Similar articles
-
Stereoselective Synthesis of 3-Oxabicyclo[3.3.1]nonan-2-ones via a Domino Reaction Catalyzed by Modularly Designed Organocatalysts.Adv Synth Catal. 2019 Jan 11;361(1):208-213. doi: 10.1002/adsc.201800987. Epub 2018 Oct 30. Adv Synth Catal. 2019. PMID: 31467501 Free PMC article.
-
Organocatalytic Asymmetric Synthesis of Functionalized 1,3,5-Triarylpyrrolidin-2-ones via an Aza-Michael/Aldol Domino Reaction.Synthesis (Stuttg). 2014 Mar 1;46(6):799-808. doi: 10.1055/s-0033-1340565. Synthesis (Stuttg). 2014. PMID: 25278634 Free PMC article.
-
Organocatalytic Oxa-Michael/Michael/Michael/Aldol Condensation Quadruple Domino Sequence: Asymmetric Synthesis of Tricyclic Chromanes.Org Lett. 2018 Feb 16;20(4):1232-1235. doi: 10.1021/acs.orglett.8b00175. Epub 2018 Feb 6. Org Lett. 2018. PMID: 29406738
-
One-pot organocatalytic enantioselective Michael/Povarov domino strategy for the construction of spirooctahydroacridine-3,3'-oxindole scaffolds.Chemistry. 2014 May 12;20(20):5899-904. doi: 10.1002/chem.201402002. Epub 2014 Mar 20. Chemistry. 2014. PMID: 24652778
-
Advances in organocatalyzed synthesis of organic compounds.RSC Adv. 2024 Jun 25;14(28):20365-20389. doi: 10.1039/d4ra03046j. eCollection 2024 Jun 18. RSC Adv. 2024. PMID: 38919284 Free PMC article. Review.
Cited by
-
Asymmetric synthesis of highly functionalized tetrahydropyrans via a one-pot organocatalytic Michael/Henry/ketalization sequence.Org Lett. 2014 Jul 18;16(14):3636-9. doi: 10.1021/ol501236a. Epub 2014 Jun 27. Org Lett. 2014. PMID: 24971998 Free PMC article.
-
Organocatalytic Asymmetric Synthesis of Dihydroisoquinolinones via a One-Pot Aza-Henry-Hemiaminalization-Oxidation Sequence.Synthesis (Stuttg). 2015 Feb 1;47(4):472-480. doi: 10.1055/s-0034-1379398. Synthesis (Stuttg). 2015. PMID: 26722132 Free PMC article.
-
Recent Advances in Asymmetric Organocatalyzed Conjugate Additions to Nitroalkenes.Molecules. 2017 May 29;22(6):895. doi: 10.3390/molecules22060895. Molecules. 2017. PMID: 28555049 Free PMC article. Review.
-
Asymmetric synthesis of tetrahydropyridines via an organocatalytic one-pot multicomponent Michael/aza-Henry/cyclization triple domino reaction.Org Lett. 2014 Nov 21;16(22):6012-5. doi: 10.1021/ol503024d. Epub 2014 Nov 7. Org Lett. 2014. PMID: 25379786 Free PMC article.
-
Stereoselective Synthesis of 3-Oxabicyclo[3.3.1]nonan-2-ones via a Domino Reaction Catalyzed by Modularly Designed Organocatalysts.Adv Synth Catal. 2019 Jan 11;361(1):208-213. doi: 10.1002/adsc.201800987. Epub 2018 Oct 30. Adv Synth Catal. 2019. PMID: 31467501 Free PMC article.
References
-
-
For reviews on organocatalytic domino reactions, see:
- Sheffler U, Mahrwald R. Chem. Eur. J. 2013;19:14346. - PubMed
- Bonne D, Constanieux T, Coquerel Y, Rodriguez J. Chem. Eur. J. 2013;19:2218. - PubMed
- Pellissier H. Chem. Rev. 2013;113:442. - PubMed
- Grossmann A, Enders D. Angew. Chem. Int. Ed. 2011;51:314. - PubMed
- Enders D, Jeanty M, Grondal C. Nat. Chem. 2010;2:167. - PubMed
- Alba A-N, Companyo X, Viciano M, Rios R. Curr. Org. Chem. 2009;13:1432.
- Yu X, Wang W. Org. Biomol. Chem. 2008;6:2037. - PubMed
- Enders D, Grondal C, Hüttl MRM. Angew. Chem. Int. Ed. 2007;46:1570. - PubMed
-
-
-
For selected general reviews on hydrogen bonding, see:
- Lu T, Wheeler SE. Chem. Eur. J. 2013;19:15141. - PubMed
- Wurm FR, Klok H-A. Chem. Soc. Rev. 2013;42:8220. - PubMed
- Brière J-F, Oudeyer S, Dalla V, Levacher V. Chem. Soc. Rev. 2012;41:1696. - PubMed
- Alemán J, Parra A, Jiang H, Jørgensen KA. Chem. Eur. J. 2011;17:6890. - PubMed
- Knowles RR, Jacobsen EN. Proc. Natl. Acad. Sci. U.S.A. 2010;107:20678. - PMC - PubMed
- Etzenbach-Effers K, Berkessel A. Top. Curr. Chem. 2009;291:1. - PubMed
- Takemoto Y. Chem. Pharm. Bull. 2010;58:593. - PubMed
- Kotke M, Schreiner PR. In: Hydrogen Bonding in Organic Synthesis. Pihko PM, editor. Wiley-VCH; Weinheim: 2009. p. 141.
- Zhang Z, Schreiner PR. Chem. Soc. Rev. 2009;38:1187. - PubMed
- Doyle AG, Jacobsen EN. Chem. Rev. 2007;107:5713. - PubMed
- Taylor MS, Jacobsen EN. Angew. Chem. Int. Ed. 2006;45:1520. - PubMed
- Schreiner PR. Chem. Soc. Rev. 2003;32:289. - PubMed
-
-
-
For reviews on tetrahydropyran syntheses, see:
- Olier C, Kaafarani M, Gastaldi S, Bertrand MP. Tetrahedron. 2010;66:413.
- Larrosa I, Romea P, Urpí F. Tetrahedron. 2008;64:2683.
- Clarke PA, Santos S. Eur. J. Org. Chem. 2006:2045.
- Shindo M. Top. Heterocycl. Chem. 2006;5:179.
- Lee E. Pure Appl. Chem. 2005;77:2073.
- Yeung K-S, Paterson I. Chem. Rev. 2005;105:4237. - PubMed
- Boivin TLB. Tetrahedron. 1987;43:3309.
-
-
-
For selected examples of tetrahydropyran syntheses, see:
- Dange NS, Hong B-C, Lan D-J, Liao J-H, Lee G-H. Eur. J. Org. Chem. 2013;12:2472.
- Wang Y, Zhu S, Ma D. Org. Lett. 2011;13:1602. - PubMed
- Lee K, Kim H, Hong J. Eur. J. Org. Chem. 2012:1025.
- Ishikawa H, Sawano S, Yasui Y, Shibata Y, Hayashi Y. Angew. Chem. Int. Ed. 2011;50:3774. - PubMed
- Liu L, Floreancig PE. Angew. Chem. Int. Ed. 2010;49:3069. - PMC - PubMed
- Fuwa H, Noto K, Sasaki M. Org. Lett. 2010;12:1636. - PubMed
- Trost BM, Gutierrez AC, Livingston RC. Org. Lett. 2009;11:2539. - PMC - PubMed
- Chandrasekhar S, Mallikarjun K, Pavankumarreddy G, Veeramohana Rao K, Jagadeesh B. Chem. Commun. 2009:4985. - PubMed
- Cao C-L, Zhou Y-Y, Sun X-L, Tang Y, Li Y-X, Li G-Y, Sun J. Chem. Eur. J. 2009;15:11384. - PubMed
- Gotoh H, Okamura D, Ishikawa H, Hayashi Y. Org. Lett. 2009;11:4056. - PubMed
- De Brab JK, Liu B, Qian M. Org. Lett. 2008;10:2533. - PubMed
- Hiebel M-A, Pelotier B, Goekjian P, Piva O. Eur. J. Org. Chem. 2008:713.
-
-
- Enders D, Urbanietz G, Raabe G. Synthesis. 2011:1905.
- Enders D, Urbanietz G, Hahn R, Raabe G. Synthesis. 2012;44:773.
- Ramachary DB, Madhavachary R, Prasad MS. Org. Biomol. Chem. 2012;10:5825. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources