Oxygen supersaturated fluid using fine micro/nanobubbles
- PMID: 25285003
- PMCID: PMC4181745
- DOI: 10.2147/IJN.S68840
Oxygen supersaturated fluid using fine micro/nanobubbles
Abstract
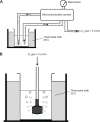
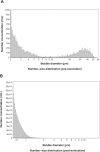
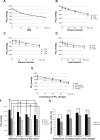
Microbubbles show peculiar properties, such as shrinking collapse, long lifetime, high gas solubility, negative electric charge, and free radical production. Fluids supersaturated with various gases can be easily generated using microbubbles. Oxygen microbubble fluid can be very useful for oxygen delivery to hypoxic tissues. However, there have been no reports of comparative investigations into adding fluids containing oxygen fine micro/nanobubbles (OFM-NBs) to common infusion solutions in daily medical care. In this study, it was demonstrated that OFMNBs can generate oxygen-supersaturated fluids, and they may be sufficiently small to infuse safely into blood vessels. It was found that normal saline solution is preferable for generating an oxygen-rich infusion fluid, which is best administered as a 30-minute intravenous infusion. It was also concluded that dextran solution is suitable for drug delivery substances packing oxygen gas over a 1-hour intravenous infusion. In addition, normal saline solution containing OFMNBs was effective for improving blood oxygenation. Thus, the use of OFMNB-containing fluids is a potentially effective novel method for improving blood oxygenation in cases involving hypoxia, ischemic diseases, infection control, and anticancer chemoradiation therapies.
Keywords: fine micro/nanobubble; fluid oxygenation; microbubble; nanobubble; oxygenation.
Figures


Similar articles
-
Blood oxygenation using microbubble suspensions.Eur Biophys J. 2012 Jun;41(6):571-8. doi: 10.1007/s00249-012-0811-y. Epub 2012 Apr 3. Eur Biophys J. 2012. PMID: 22476882
-
Oxygen-Carrying Micro/Nanobubbles: Composition, Synthesis Techniques and Potential Prospects in Photo-Triggered Theranostics.Molecules. 2018 Aug 31;23(9):2210. doi: 10.3390/molecules23092210. Molecules. 2018. PMID: 30200336 Free PMC article. Review.
-
Ultrasound beam steering of oxygen nanobubbles for enhanced bladder cancer therapy.Sci Rep. 2018 Feb 15;8(1):3112. doi: 10.1038/s41598-018-20363-8. Sci Rep. 2018. PMID: 29449656 Free PMC article.
-
Simple method to make a supersaturated oxygen fluid.J Artif Organs. 2018 Sep;21(3):392-395. doi: 10.1007/s10047-018-1022-9. Epub 2018 Jan 22. J Artif Organs. 2018. PMID: 29356911
-
Role of Solid-Gas Interface of Nanobubbles for Therapeutic Applications.Crit Rev Ther Drug Carrier Syst. 2018;35(5):469-494. doi: 10.1615/CritRevTherDrugCarrierSyst.2018020229. Crit Rev Ther Drug Carrier Syst. 2018. PMID: 30317946 Review.
Cited by
-
Oxygen delivery to the blood stream by the dialysis fluid during continuous renal replacement therapy ex vivo.J Artif Organs. 2019 Jun;22(2):104-109. doi: 10.1007/s10047-018-1085-7. Epub 2019 Jan 2. J Artif Organs. 2019. PMID: 30603819
-
Anti-Tumor Drug-Loaded Oxygen Nanobubbles for the Degradation of HIF-1α and the Upregulation of Reactive Oxygen Species in Tumor Cells.Cancers (Basel). 2019 Sep 29;11(10):1464. doi: 10.3390/cancers11101464. Cancers (Basel). 2019. PMID: 31569523 Free PMC article.
-
Topical oxygen therapy & micro/nanobubbles: a new modality for tissue oxygen delivery.Int Wound J. 2018 Jun;15(3):363-374. doi: 10.1111/iwj.12873. Epub 2018 Jan 5. Int Wound J. 2018. PMID: 29314626 Free PMC article. Review.
-
Cerebrospinal fluid oxygen optimisation for rescue of metabolically challenged in vitro cortical brain tissue.IBRO Rep. 2020 Nov 4;9:302-309. doi: 10.1016/j.ibror.2020.10.007. eCollection 2020 Dec. IBRO Rep. 2020. PMID: 33235940 Free PMC article.
-
Current advances in ultrasound-combined nanobubbles for cancer-targeted therapy: a review of the current status and future perspectives.RSC Adv. 2021 Apr 6;11(21):12915-12928. doi: 10.1039/d0ra08727k. eCollection 2021 Mar 29. RSC Adv. 2021. PMID: 35423829 Free PMC article. Review.
References
-
- Wujtewicz M. Fluid use in adult intensive therapy. Anesthesiol Intensive Ther. 2012;44(2):92–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

