Redefining Human Vitamin D Sufficiency: Back to the Basics
- PMID: 25285234
- PMCID: PMC4180627
- DOI: 10.4248/BR201301002
Redefining Human Vitamin D Sufficiency: Back to the Basics
Abstract
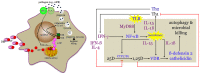
The role of the endocrine vitamin D pathway in regulating the serum calcium concentration in man is well described. In the presence of a low serum calcium level, the vitamin D metabolic pathway is called upon to produce more of the active vitamin D hormone, 1, 25-dihydroxyvitamin D (1, 25-D), via up-regulation of the CYP27b1-hydroxylase activity in the kidney. The consequence is mobilization of skeletal calcium stores to return the circulating calcium level back to the normal range. On the other hand, when the serum calcium level increases, endocrine forces move to suppress renal 1, 25D production and squelch bone resorption. It is now known that vitamin D metabolites also operate in an autocrine, paracrine and intracrine mode to control vitamin D receptor-directed biological responses at the tissue level. Because the metabolism and action of vitamin D occurs at the tissue level in this situation, use of circulating vitamin D metabolites and biomarkers to ascertain the local action of the vitamin D pathway is often misleading. This mini-review seeks to compare and contrast the operation of the vitamin D pathway at the endocrine and tissue level.
Figures

References
-
- Zhu J, DeLuca HF. Vitamin D 25-hydroxylase—Four decades of searching, are we there yet? Arch Biochem Biophys. 2012;523:30–36. - PubMed
-
- Endres DB. Investigation of hypercalcemia. Clin Biochem. 2012;45:954–963. - PubMed
-
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zügel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

