A randomized controlled trial of cholecalciferol supplementation in patients on maintenance hemodialysis
- PMID: 25285282
- PMCID: PMC4171888
- DOI: 10.4103/2230-8210.139227
A randomized controlled trial of cholecalciferol supplementation in patients on maintenance hemodialysis
Abstract
Background: Vitamin D deficiency is common in Indian patients with chronic kidney disease (CKD) on maintenance hemodialysis (MHD), but optimal dose of cholecalciferol is unclear.
Materials and methods: A total of 45 consenting patients were randomized to intervention and control groups. In the intervention group, patients (n = 35) with serum 25-hydroxy vitamin D (25(OH)D) < 30 ng/mL (n = 33), received oral cholecalciferol 60,000 units/week for 6 weeks. The serum levels of 25(OH)D, calcium, phosphorus, albumin, and parathyroid hormone (PTH) were measured at 0, 6, and 12 weeks. In the control group (n = 10), these were estimated at 0 and 6 weeks.
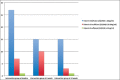
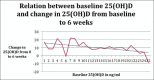
Results: In the intervention group, 25/35 patients completed the supplementation at 6 weeks and 20/35 were available at 12 weeks. The mean baseline level of 25(OH)D was 9.59 ± 7.59 ng/mL, and after 6 weeks 19.51 ± 4.27 ng/mL, mean increase being 9.99 ± 6.83 ng/mL, which was highly significant (P < 0.0001). After discontinuing supplementation at 6 weeks, serum 25(OH)D level dropped significantly from 6 to 12 weeks [-2.84 ± 6.25 ng/mL (P = 0.04)]. However, it was still significantly higher at 12 weeks (16.08 ± 8.27 ng/mL) as compared with the baseline. PTH and calcium did not change significantly with supplementation. The change in serum 25(OH)D level from baseline to 6 weeks in the intervention group was inversely related to baseline 25(OH)D levels and patient's weight. In the control group, change in 25(OH)D from baseline to 6 weeks was not significant.
Conclusion: Supplementation with cholecalciferol 60,000 unit/week for 6 weeks was insufficient to achieve optimal levels of 25(OH)D in Indian patients with CKD on MHD.
Keywords: Cholecalciferol; India; chronic kidney disease; hemodialysis; vitamin D hemodialysis.
Conflict of interest statement
Figures
Similar articles
-
Biochemical parameters after cholecalciferol repletion in hemodialysis: results From the VitaDial randomized trial.Am J Kidney Dis. 2014 Nov;64(5):696-705. doi: 10.1053/j.ajkd.2014.04.020. Epub 2014 May 22. Am J Kidney Dis. 2014. PMID: 24856872 Clinical Trial.
-
[Cholecalciferol supplementation improves secondary hyperparathyroidism control in hemodialysis patients].G Ital Nefrol. 2020 Jun 10;37(3):2020-vol3. G Ital Nefrol. 2020. PMID: 32530156 Italian.
-
[Changes in mineral metabolism in stage 3, 4, and 5 chronic kidney disease (not on dialysis)].Nefrologia. 2008;28 Suppl 3:67-78. Nefrologia. 2008. PMID: 19018742 Spanish.
-
Effect of two different doses of oral cholecalciferol supplementation on serum 25-hydroxy-vitamin D levels in healthy Indian postmenopausal women: A randomized controlled trial.Indian J Endocrinol Metab. 2013 Sep;17(5):883-9. doi: 10.4103/2230-8210.117237. Indian J Endocrinol Metab. 2013. PMID: 24083171 Free PMC article.
-
1alpha(OH)D3 One-alpha-hydroxy-cholecalciferol--an active vitamin D analog. Clinical studies on prophylaxis and treatment of secondary hyperparathyroidism in uremic patients on chronic dialysis.Dan Med Bull. 2008 Nov;55(4):186-210. Dan Med Bull. 2008. PMID: 19232159 Review.
Cited by
-
Analysis of the parathyroid function in maintenance hemodialysis patients from Changchun, China.Chronic Dis Transl Med. 2017 Aug 7;3(3):181-185. doi: 10.1016/j.cdtm.2017.07.001. eCollection 2017 Sep. Chronic Dis Transl Med. 2017. PMID: 29063075 Free PMC article.
-
Clinical practice recommendations for native vitamin D therapy in children with chronic kidney disease Stages 2-5 and on dialysis.Nephrol Dial Transplant. 2017 Jul 1;32(7):1098-1113. doi: 10.1093/ndt/gfx065. Nephrol Dial Transplant. 2017. PMID: 28873969 Free PMC article.
-
Cholecalciferol Additively Reduces Serum Parathyroid Hormone Levels in Severe Secondary Hyperparathyroidism Treated with Calcitriol and Cinacalcet among Hemodialysis Patients.Nutrients. 2018 Feb 10;10(2):196. doi: 10.3390/nu10020196. Nutrients. 2018. PMID: 29439405 Free PMC article. Clinical Trial.
-
Cholecalciferol Additively Reduces Serum Parathyroid Hormone and Increases Vitamin D and Cathelicidin Levels in Paricalcitol-Treated Secondary Hyperparathyroid Hemodialysis Patients.Nutrients. 2016 Nov 5;8(11):708. doi: 10.3390/nu8110708. Nutrients. 2016. PMID: 27827962 Free PMC article. Clinical Trial.
References
-
- Nigwekar SU, Bhan I, Thadhani R. Ergocalciferol and cholecalciferol in CKD. Am J Kidney Dis. 2012;60:139–56. - PubMed
-
- Reichel H, Koeffler HP, Norman AW. Synthesis in vitro of 1,25-dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3 by interferon-gamma-stimulated normal human bone marrow and alveolar macrophages. J Biol Chem. 1987;262:10931–7. - PubMed
-
- Hewison M, Burke F, Evans KN, Lammas DA, Sansom DM, Liu P, et al. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103:316–21. - PubMed
-
- NKF/KDOQI Clinical Practice guidelines for bone metabolism and disease in chronic kidney disease. [Last accessed on 2010 Sep 3]. Available from: http://www.kidney.org/professionals/KDOQI/guidelines_bone/index.htm .

