Visibly emissive and responsive extended 6-aza-uridines
- PMID: 25285451
- PMCID: PMC4201329
- DOI: 10.1021/ol502435d
Visibly emissive and responsive extended 6-aza-uridines
Abstract
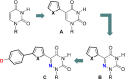
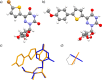
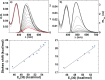
A family of extended 5-modified-6-aza-uridines was obtained via Suzuki coupling reactions with a common brominated precursor. Extending the conjugated-6-aza-uridines with substituted aryl rings increases the push-pull interactions yielding enhanced bathochromic shifts and solvatochromism compared to the parent nucleosides. For example, the methoxy substituted derivative 1d displays λmax abs around 375 nm, with visible emission maxima at 486 nm (Φ = 0.74) and 525 nm (Φ = 0.02) in dioxane and water, respectively.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

